Answered step by step
Verified Expert Solution
Question
1 Approved Answer
answer the question fully and don't copy someone elses answer or you will be reported for spam. Problem 3 (45 pts) Extraction of acetone by
answer the question fully and don't copy someone elses answer or you will be reported for spam. 
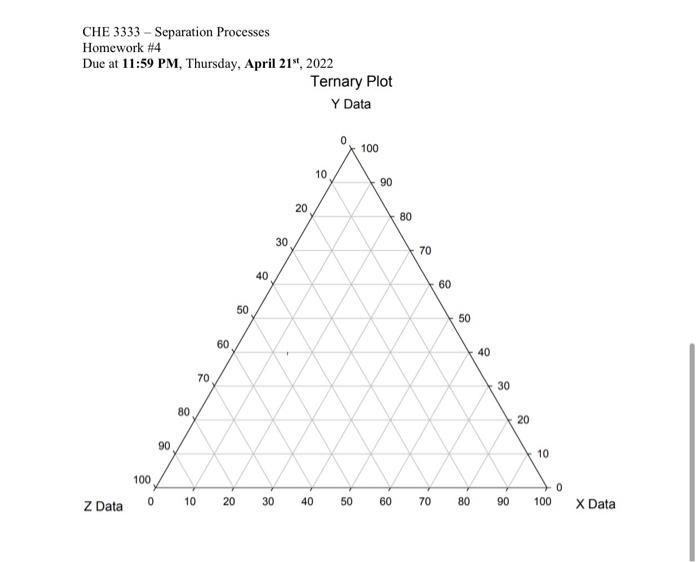
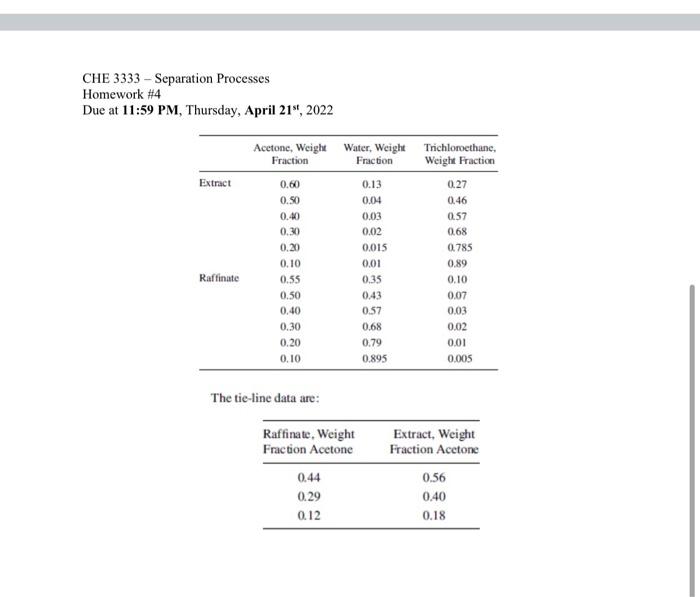
Problem 3 (45 pts) Extraction of acetone by trichloroethane (TCE) 1000 kg/h of a 45 wt% acetone-in-water solution is to be extracted at 25C in a continuous, countercurrent system with pure 1,1,2-trichloroethane (TCE) to obtain a raffinate containing 10 wt% acetone. Using the following equilibrium data, determine with an equilateral-triangle diagram (10p): (a) the minimum flow rate of solvent (10p) (b) the number of stages required for a solvent rate equal to 1.5 times minimum (10p) (c) the flow rate and composition of each stream leaving each stage (15p) CHE 3333 - Separation Processes Homework #4 Due at 11:59 PM, Thursday, April 21", 2022 Ternary Plot Y Data 100 10 90 20 80 30 70 40 60 50 50 60 40 70 30 80 20 90 10 100 o 0 10 20 30 40 50 60 70 80 90 100 Z Data X Data CHE 3333 - Separation Processes Homework #4 Due at 11:59 PM, Thursday, April 21", 2022 Extract 0.04 0.30 Acetone, Weight Water Weight Trichloroethane, Fraction Fraction Weight Fraction 0.60 0.13 0.27 0.50 046 0.40 0.03 0.57 0.02 0.68 0.20 0,015 0.785 0.10 0.01 0.89 0.55 0.35 0.10 0.50 0.43 0.07 0.57 0.03 0.30 0.68 0.02 0.79 0.01 0.10 0.895 0.005 Raffinate 0.40 0.20 The tie-line data are: Raffinate, Weight Fraction Acetone 0.44 0.29 0.12 Extract, Weight Fraction Acetone 0.56 0.40 0.18 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


