Answer the questions in the last picture
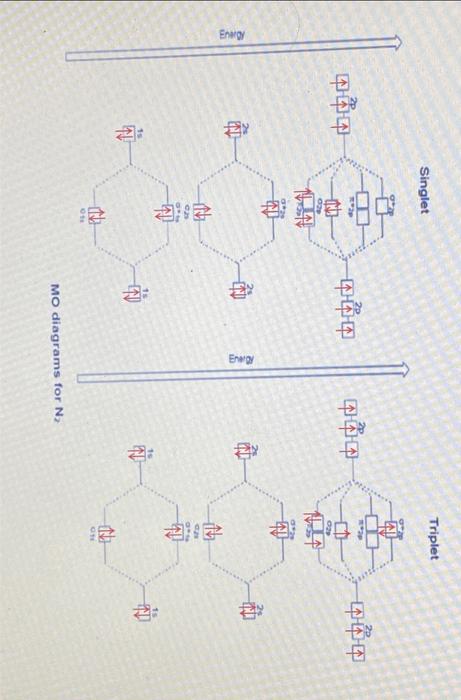
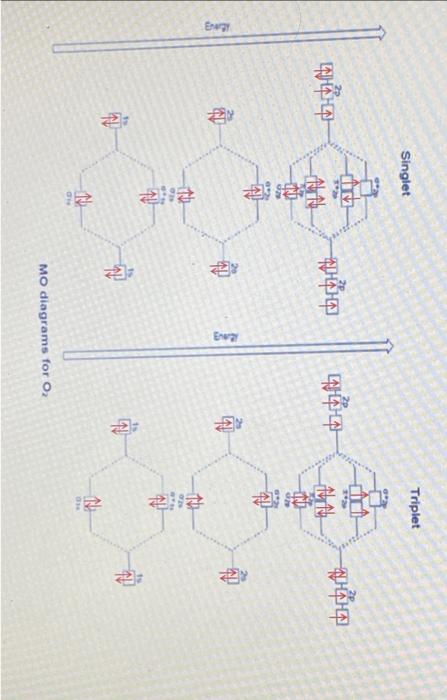
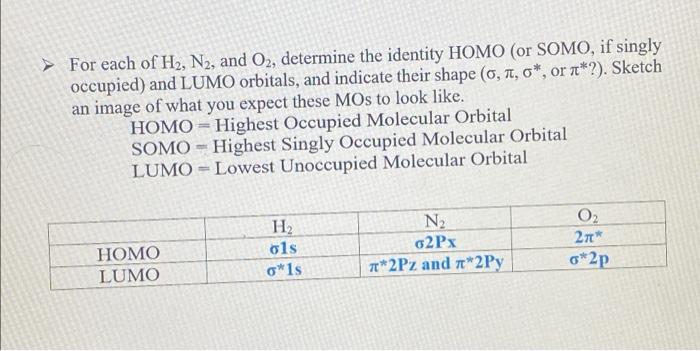
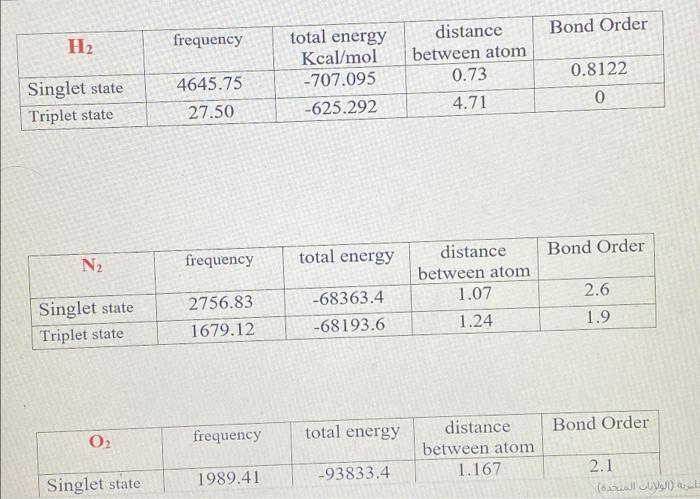
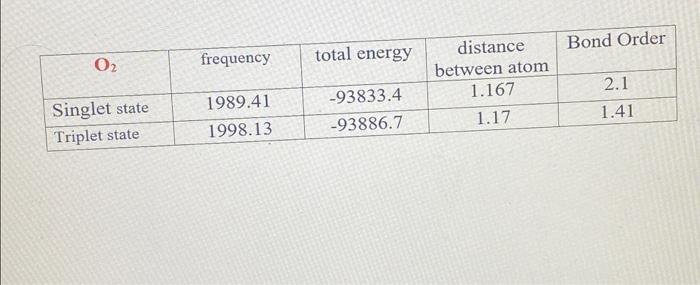
Lab3: Molecular Orbitals of Some Diatomic Molecules A. Molecular orbitals of H2,N2, and O2 B. Electron density of HF and LiH molecules A- Molecular orbitals of H2,N2, and O2 In this lab we will focus on three diatomic molecules (which at standard conditions exist as well-known gases-hydrogen, nitrogen, and oxygen): H2, N2, and O2. The singlet and triplet electronic configurations of H2 were already presented in the introduction. Use the MO diagram below to fill out the electronic configuration of N2 in its lowest singlet and triplet state. Follow Hund's rule and the "building-up" principle when possible but make sure that the Pauli exclusion principle is always enforced. Mo atagrams for N2 Similarly, do the same for O2 using the MO diagram on the following page. Note that in O2 (as well as F2, etc.), there is a reordering of the 2p and 2p orbitals compared to N2 and smaller diatomics. The reason for this is mixing between the 2s and 2p molecular orbitals in the smaller diatomics which causes the 2p MO to increase in energy. ams for O2 For each of H2,N2, and O2, determine the identity HOMO (or SOMO, if singly occupied) and LUMO orbitals, and indicate their shape (,,, or ?). Sketch an image of what you expect these MOs to look like. HOMO= Highest Occupied Molecular Orbital SOMO = Highest Singly Occupied Molecular Orbital LUMO = Lowest Unoccupied Molecular Orbital Identify the formal bond order of each molecule in its lowest singlet and triplet states. Which state (the singlet or the triplet) has a more energetically favorable configuration based on the rules discussed in the introduction? Bond Order =1/2 ((Number of bonding electrons) - (Number of antibonding electrons)) N2 singlet 1/2(104)=3 N2 triplet 21(86)=1 singlet 1/2(106)=2 triplet 1/2(106)=2 aint \begin{tabular}{|l|c|c|c|c|} \hline \multicolumn{1}{|c|}{O2} & frequency & total energy & distance between atom & Bond Order \\ \hline Singlet state & 1989.41 & 93833.4 & 1.167 & 2.1 \\ \hline Triplet state & 1998.13 & 93886.7 & 1.17 & 1.41 \\ \hline \end{tabular} 1. Is there a relation between the bond distances computed in IQmol and your bond orders predicted from MO theory for each molecule in each state? Explain your answer. 2. Compare the singlet and triplet state energies for each molecule. Which state is lower in energy, the singlet or the triplet? Note that lower in energy means more negative. Is this consistent with your expectations from your MO diagram? Explain which rule plays the main role in determining the ground state multiplicity of O2. 3. Compare your computed HOMO and LUMO orbitals with the sketches you drew after constructing the MO diagrams. Are they consistent with what you expected? Explain. 4. How many nodes does the HOMO have in H2,N2, and O2 ? Does the number of nodes change when changing states for each molecule? Explain your