Answered step by step
Verified Expert Solution
Question
1 Approved Answer
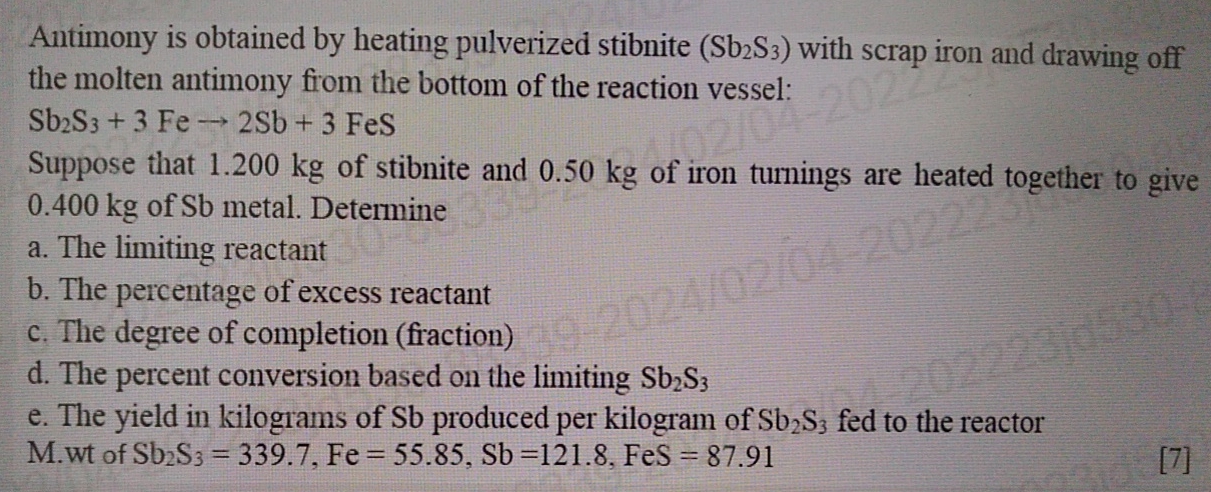
Antimony is obtained by heating pulverized stibnite ( S b 2 S 3 ) with scrap iron and drawing off the molten antimony from the
Antimony is obtained by heating pulverized stibnite with scrap iron and drawing off the molten antimony from the bottom of the reaction vessel:
FeS
Suppose that of stibnite and of iron turnings are heated together to give of metal. Determine
a The limiting reactant
b The percentage of excess reactant
c The degree of completion fraction
d The percent conversion based on the limiting
e The yield in kilograms of produced per kilogram of fed to the reactor Mwt of FeS
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


