Answered step by step
Verified Expert Solution
Question
1 Approved Answer
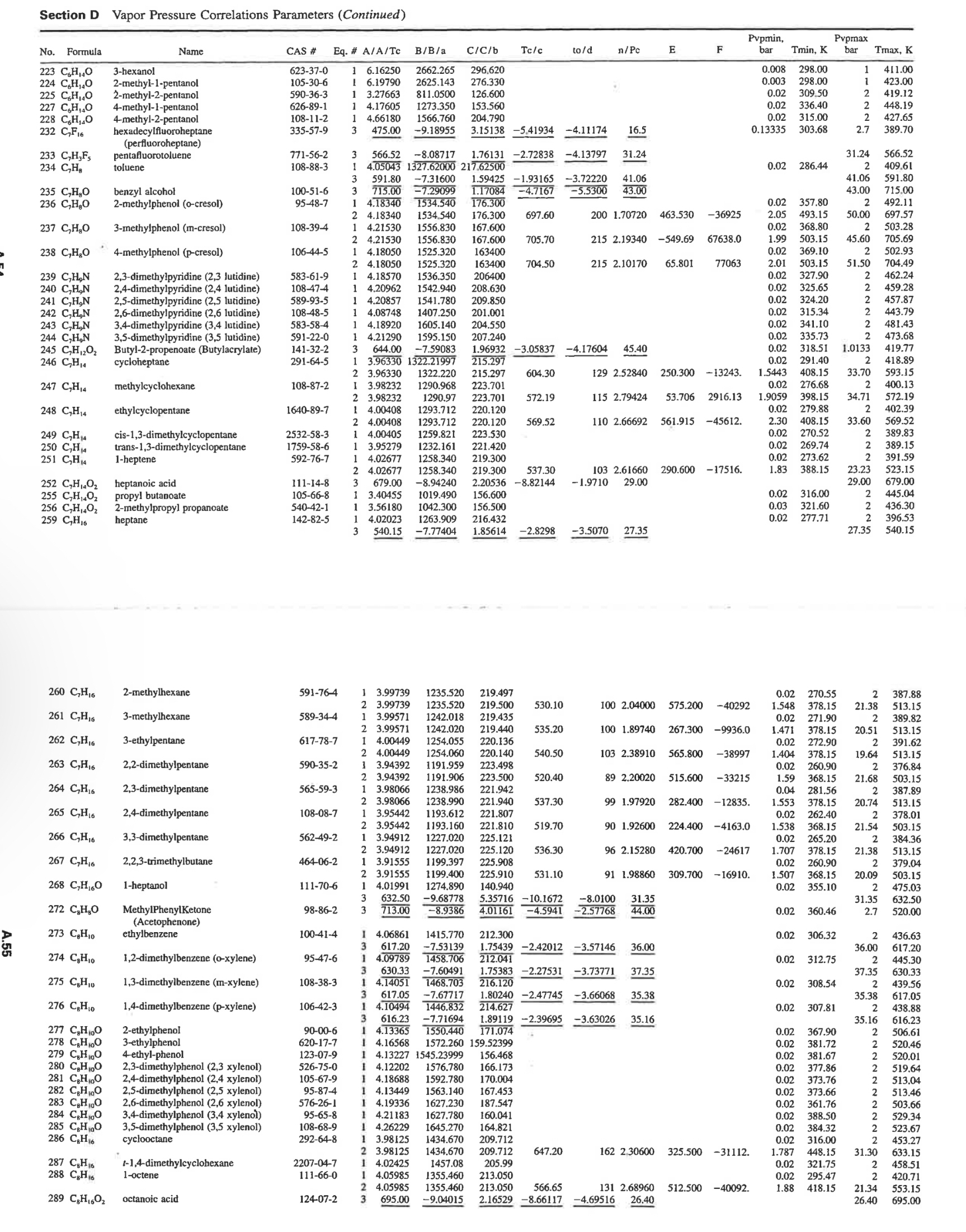
Antoine's equation is a correlation for vapor pressures based on the ClausiusClapeyron equation. It has been used over limited temperature ranges. l o g 1
Antoine's equation is a correlation for vapor pressures based on the ClausiusClapeyron equation. It has been used over limited temperature ranges.
where is in and Pvap is in bar. Use Page A of Poling et al for the coefficients needed for ethylbenzene.
a Calculate the vapor pressure of ethylbenzene at and with Antoine's equation.
b
For the above, experimental values are bar at Chaiyavech and van Winkle and bar at Ambrose et al respectively. For each temperature, report the relative deviation using the following equation:
c
A more elaborate method is a modified Wagner equation
where is the reduced vapor pressure is the reduced temperature, and is calculate the vapor pressure of ethylbenzene at and with the Wagner equation. Based on the experimental data given in part report the relative deviation for each data point.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


