Question
any 1. Solve using (a) bisection method and (b) newton-raphson. Use initial guess. (10pts.) The ideal gas law is given by PV = RT,
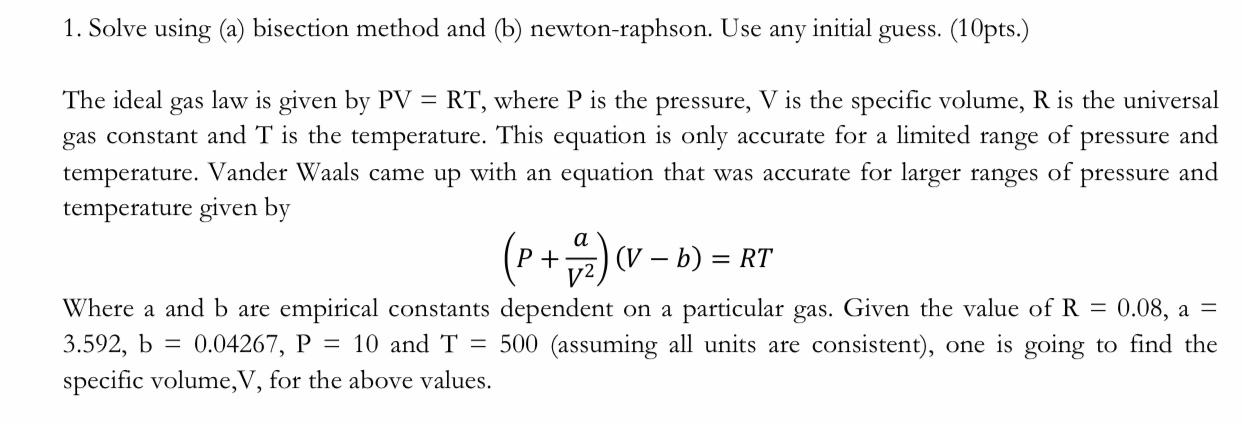
any 1. Solve using (a) bisection method and (b) newton-raphson. Use initial guess. (10pts.) The ideal gas law is given by PV = RT, where P is the pressure, V is the specific volume, R is the universal gas constant and T is the temperature. This equation is only accurate for a limited range of pressure and temperature. Vander Waals came up with an equation that was accurate for larger ranges of pressure and temperature given by a (P+) (V b) = RT 0.08, 3 Where a and b are empirical constants dependent on a particular gas. Given the value of R = 3.592, b = 0.04267, P = 10 and T = 500 (assuming all units are consistent), one is going to find the specific volume,V, for the above values.
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



