Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Any help would be greatly apprecitaed thank you! please include MATLAB code Ethylene oxide (C) can be produced by the vapor-phase catalytic oxidation of ethylene
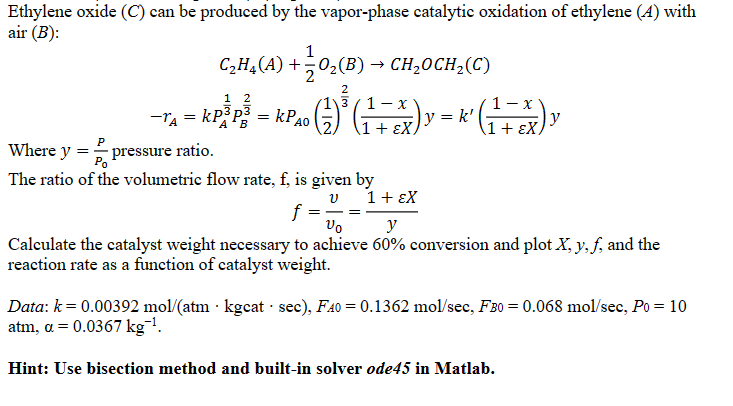
Any help would be greatly apprecitaed thank you! please include MATLAB code
Ethylene oxide (C) can be produced by the vapor-phase catalytic oxidation of ethylene (A) with air (B) : C2H4(A)+21O2(B)CH2OCH2(C)rA=kPA31PB32=kPA0(21)32(1+X1x)y=k(1+X1x)y Where y=P0P pressure ratio. The ratio of the volumetric flow rate, f, is given by f=v0v=y1+X Calculate the catalyst weight necessary to achieve 60% conversion and plot X,y,f, and the reaction rate as a function of catalyst weight. Data: k=0.00392mol/(atmkgcatsec),FA=0.1362mol/sec,FB0=0.068mol/sec,P0=10 atm,=0.0367kg1 Hint: Use bisection method and built-in solver ode45 in MatlabStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


