Answered step by step
Verified Expert Solution
Question
1 Approved Answer
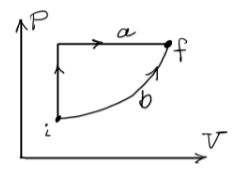
Any point on the thermodynamic P V diagram below is an equilibrium state. An unknown process, not shown on the diagram below, takes a system
Any point on the thermodynamic P V diagram below is an equilibrium state. An unknown
process, not shown on the diagram below, takes a system from equilibrium state i to equilibrium state f
These two equilibrium states, and two paths between them, are shown on the P V diagram. Determine
whether the statements below are true or false and give a brief reason for your judgment.
a As the process may not be reversible, that is may not be through equilibrium states, the change in
internal energy, Uf Ui can not be determined.
b The work Vf
Vi P V dV along path a is greater than the work along along path b
c The change in internal energy along path a is greater than the change in internal energy along path
b
d The process a shown in the gure is reversible.
e If we further learn that the initial and nal states are well described by the ideal gas equation of
state, then the temperature of state f is greater than the temperature of state i
f All cyclic adiabatic processes have zero work ux
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


