Answered step by step
Verified Expert Solution
Question
1 Approved Answer
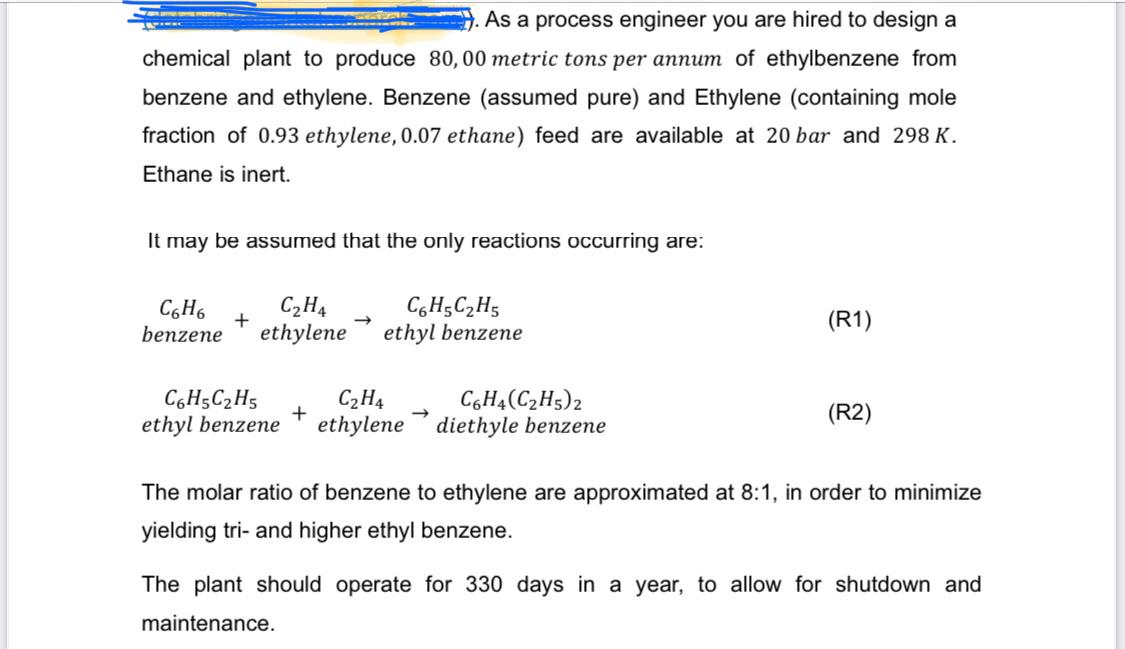
As a process engineer you are hired to design a chemical plant to produce 8 0 , 0 0 metric tons per annum of ethylbenzene
As a process engineer you are hired to design a
chemical plant to produce metric tons per annum of ethylbenzene from
benzene and ethylene. Benzene assumed pure and Ethylene containing mole
fraction of ethylene, ethane feed are available at and
Ethane is inert.
It may be assumed that the only reactions occurring are:
The molar ratio of benzene to ethylene are approximated at : in order to minimize
yielding tri and higher ethyl benzene.
The plant should operate for days in a year, to allow for shutdown and
maintenance. Executive summary
Table of Contents
Design Basis
Literature review
Process Simulation
Include process flow diagram from COCO in GREEN colour to show
you have run it
Process Description
Process Flow Diagram
Include process flow diagram with all equipment labelled tag
namenumber and stream namesnumbers and table of mass and
energy balance. Please use Microschs Visio or other software to draw
the process flow diagram. Not COCO process flow diagram.
Equipment list
Conclusions
References
Appendices if any The economics information:
Useful information:
tableUtility costs.,US DollarsLow Pressure Steam kPa saturated$GJMedium Pressure Steam kPa saturated$GJHigh Pressure Steam kPa saturated$GNatural Gas kPa,deg C$GJElectricity$kWhBoiler Feed Water at :kPa,deg C$kgCooling Water kPa: and :deg CReturn pressure kPa,Return temperature is no more than deg C above the inlet,temperature$GJRefrigerated Water available at kPa and :deg CReturn pressure kPa,Return temperature in no higher than deg C$GJLowtemperature Refrigerant, available at deg C$GJVerylowtemperature Refrigerant, available at deg C$GJDesign basis.
Literature Survey.
Please remember to properly use referencing in your report. Use Harvard
referencing.
Process Simulation COCO Include the green PFD from COCO.
Remember, the purpose of the simulator is to assist you in doing your work, not
to do the work for you. Remember to include a discussion of the manual
calculations you performed in addition to a description of your simulation and
thermodynamic property method and models used. The simulation report
generated by the simulation package can be included in the appendix.
Process Description.
Process Flow diagram with a table containing mass and energy balances: the
flowrates, compositions, temperature, and pressures of all the process streams
not needed for the utilities like steam and cooling water All the process
equipment should be indicated on this flow diagram.
Equipment list please DONT USE GPTCHAT ND PLEASE IT IN WORD FORM SHOW ALL THE CALCULATIONS AND GRAPHS I ALSO INCLUDE THE PICTURE WHERE YOU GET ALL THE INFORMATIONS THANKS As a process engineer you are hired to design a
chemical plant to produce metric tons per annum of ethylbenzene from
benzene and ethylene. Benzene assumed pure and Ethylene containing mole
fraction of ethylene, ethane feed are available at and
Ethane is inert.
It may be assumed that the only reactions occurring are:
The molar ratio of benzene to ethylene are approximated at : in order to minimize
yielding tri and higher ethyl benzene.
The plant should operate for days in a year, to allow for shutdown and
maintenance.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


