Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At 1 atm, how much energy is required to heat 47.0 g HO(s) at -24.0 C to HO(g) at 167.0 C? Use the heat
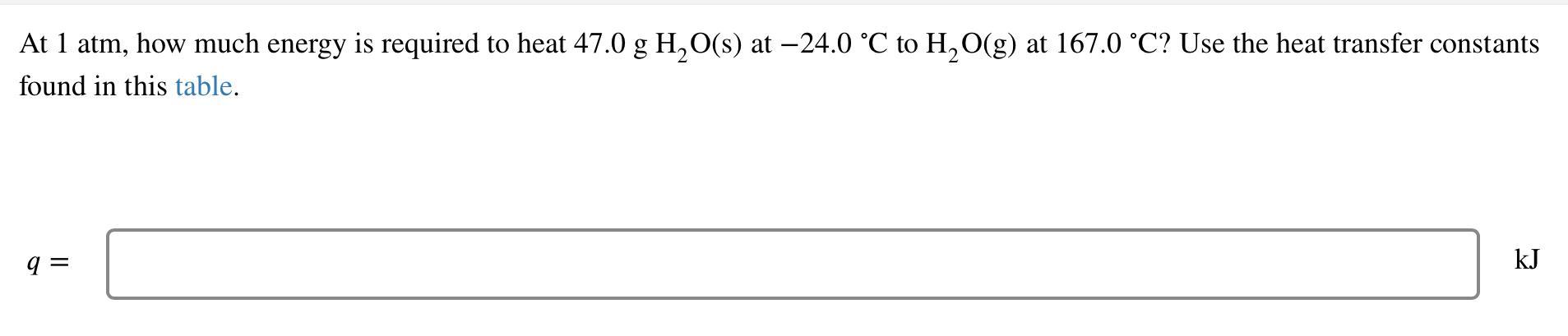

At 1 atm, how much energy is required to heat 47.0 g HO(s) at -24.0 C to HO(g) at 167.0 C? Use the heat transfer constants found in this table. q= kJ A 0.532 g sample of steam at 103.5 C is condensed into a container with 5.40 g of water at 16.0 C. What is the final temperature of the water mixture if no heat is lost? The specific heat of water is 4.18 J the specific heat of steam is g. C' 2.01 and , = 40.7 kJ/mol. vap Tf= J g. C' C
Step by Step Solution
There are 3 Steps involved in it
Step: 1
0 We have to NOCO Solid State to Step I Step III H is heat required to change 47 gm ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


