Answered step by step
Verified Expert Solution
Question
1 Approved Answer
As shown below, when 5 -deutero-5-methyl-1.3-cyclogentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as
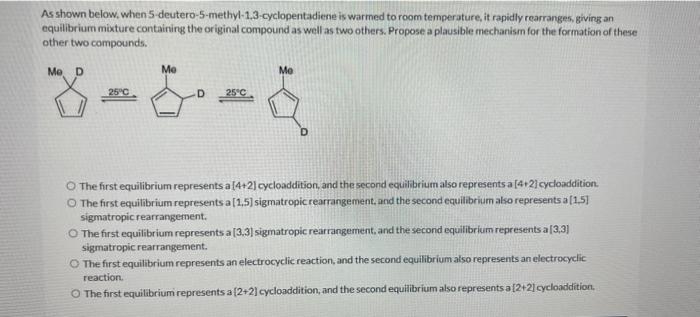 As shown below, when 5 -deutero-5-methyl-1.3-cyclogentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as two others. Propose a plausible mechanism for the formation of these other two compounds. 25C 25C. The first equilibrium represents a [4+2] cycloaddition, and the second equilibrium also represents a [4+2] cycloaddition. The first equilibrium represents a [ 1,5] sigmatropic rearrangement, and the second equilibrium akso represents a[1.5] sigmatropic rearrangement. The first equilibrium represents a [3.3] sigmatropic rearrangement, and the second equilibrium represents a[3,3] sigmatropic rearrangement. The first equilibrium represents an electrocyclic reaction, and the second equilibrium also represents an electrocyclic reaction. The first equilibrium represents a {2+2} cycloaddition, and the second equitibrium also represents a [2+2} cycloaddition
As shown below, when 5 -deutero-5-methyl-1.3-cyclogentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as two others. Propose a plausible mechanism for the formation of these other two compounds. 25C 25C. The first equilibrium represents a [4+2] cycloaddition, and the second equilibrium also represents a [4+2] cycloaddition. The first equilibrium represents a [ 1,5] sigmatropic rearrangement, and the second equilibrium akso represents a[1.5] sigmatropic rearrangement. The first equilibrium represents a [3.3] sigmatropic rearrangement, and the second equilibrium represents a[3,3] sigmatropic rearrangement. The first equilibrium represents an electrocyclic reaction, and the second equilibrium also represents an electrocyclic reaction. The first equilibrium represents a {2+2} cycloaddition, and the second equitibrium also represents a [2+2} cycloaddition

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


