Answered step by step
Verified Expert Solution
Question
1 Approved Answer
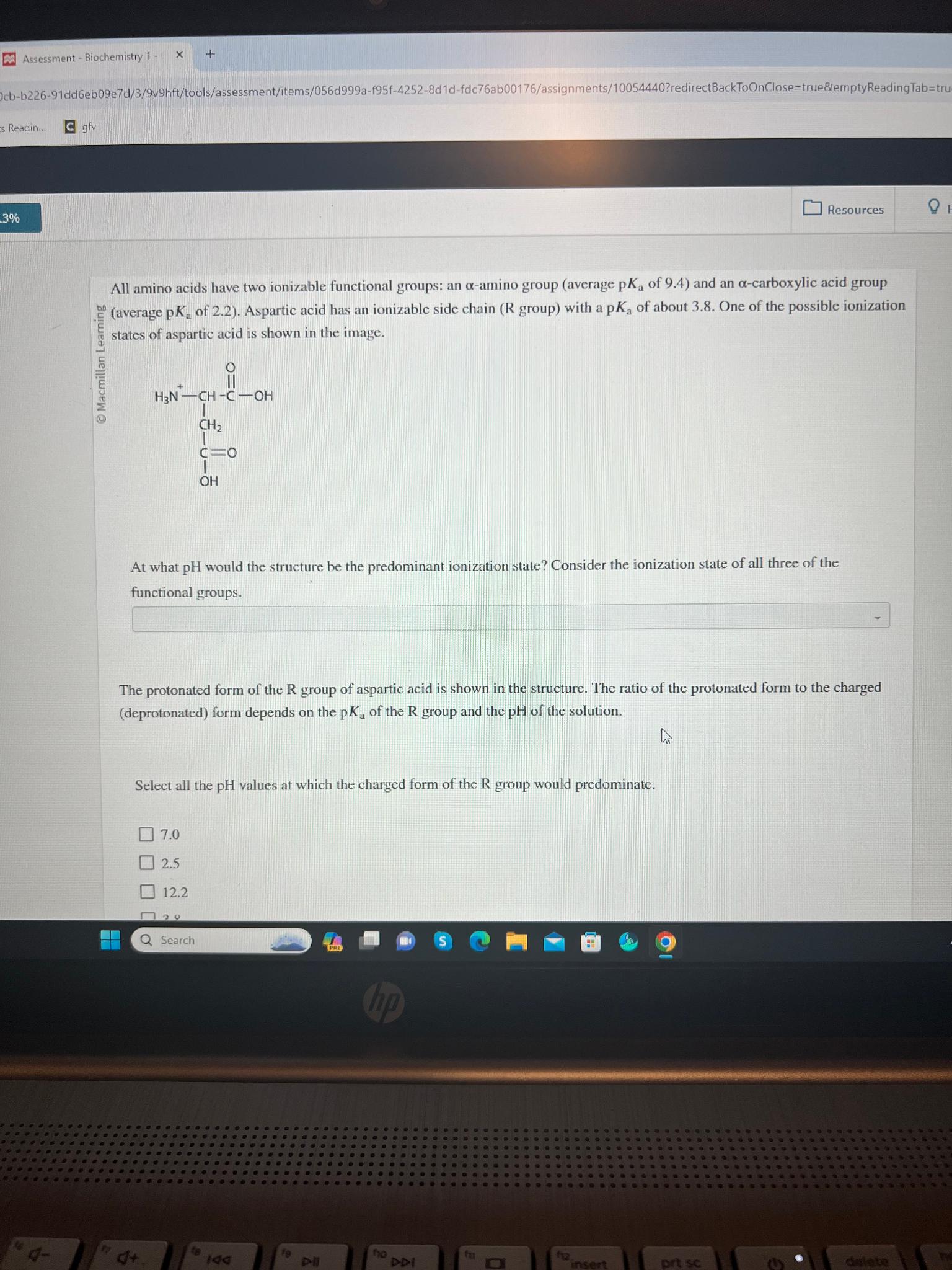
Assessment - Biochemistry 1 . s Readin... C giv Resources All amino acids have two ionizable functional groups: an - amino group ( average p
Assessment Biochemistry
s Readin...
C giv
Resources
All amino acids have two ionizable functional groups: an amino group average of and an carboxylic acid group states of aspartic acid is shown in the image.
At what would the structure be the predominant ionization state? Consider the ionization state of all three of the functional groups.
The protonated form of the group of aspartic acid is shown in the structure. The ratio of the protonated form to the charged deprotonated form depends on the of the group and the of the solution.
Select all the values at which the charged form of the group would predominate.
Search
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


