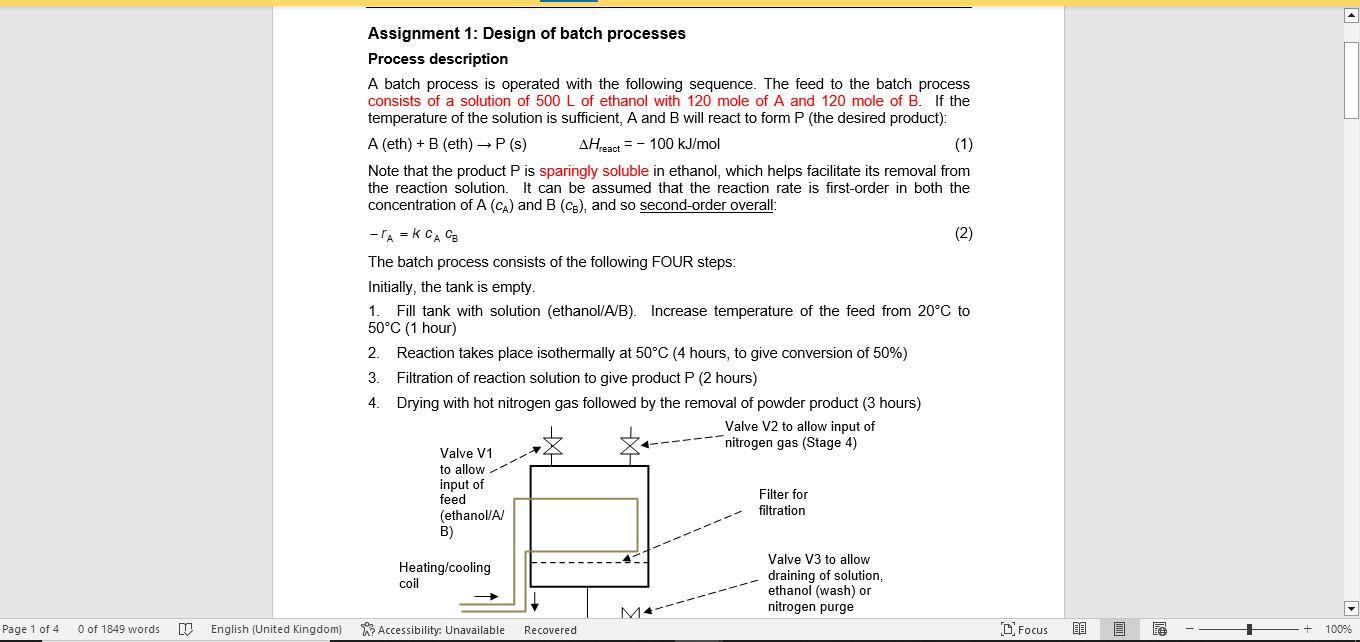
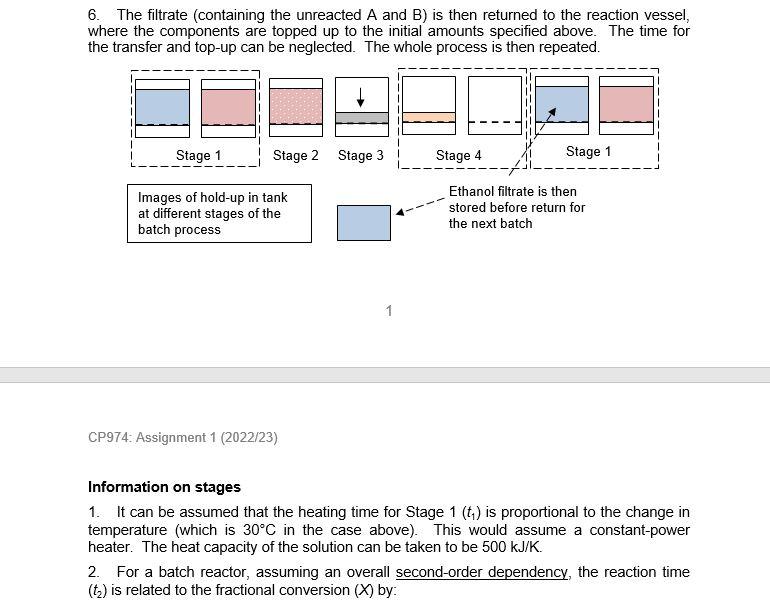


Assignment 1: Design of batch processes Process description A batch process is operated with the following sequence. The feed to the batch process consists of a solution of 500L of ethanol with 120 mole of A and 120 mole of B. If the temperature of the solution is sufficient, A and B will react to form P (the desired product): A(eth)+B(eth)P(s)Hreact=100kJ/mol Note that the product P is sparingly soluble in ethanol, which helps facilitate its removal from the reaction solution. It can be assumed that the reaction rate is first-order in both the concentration of A(cA) and B(CB), and so second-order overall: rA=kcAcB The batch process consists of the following FOUR steps: Initially, the tank is empty. 1. Fill tank with solution (ethanol/A/B). Increase temperature of the feed from 20C to 50C (1 hour) 2. Reaction takes place isothermally at 50C (4 hours, to give conversion of 50% ) 3. Filtration of reaction solution to give product P ( 2 hours) 4. Drying with hot nitrogen gas followed by the removal of powder product ( 3 hours) 6. The filtrate (containing the unreacted A and B ) is then returned to the reaction vessel, where the components are topped up to the initial amounts specified above. The time for the transfer and top-up can be neglected. The whole process is then repeated. CP974: Assignment 1(2022/23) Information on stages 1. It can be assumed that the heating time for Stage 1(t1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (t2) is related to the fractional conversion (X) by: Information on stages 1. It can be assumed that the heating time for Stage 1(t1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (t2) is related to the fractional conversion (X) by: t2=k(cA)01(1XX) where k is the rate constant (which increases with temperature) and (cA)0 is the initial concentration of component A. At the completion of the reaction, the final concentration and amount of product are (cP)end and (nP)end respectively. 3. For batch filtration, the filtration time (t3) is defined as the time taken for the entire solvent to pass through the filter case, which will increase with volume of filtrate. As the filter cake increases in depth, so does the resistance to flow which will give a reduction in volumetric flowrate. From Darcy's law and constant P filtration, it can be shown that: t3(np)endVend The final filtrate ( Vend ) is assumed to be 500L for the above case. As an example, if the volume is doubled, keeping the concentration the same, the filtration time will be quadrupled. 4. Because an excess amount of hot gas is used - the product should be bone dry for quality control purposes - the drying time (t4) can be considered to be independent of the final cake thickness, and the large value is for product quality reasons. 5. For ethanol, the maximum solubility values are 0.6mol/L and 0.25mol/L for components A and B respectively at 50C. These are the maximum amounts that will dissolve in the solvent. If more A and B is added, this will remain in the solid phase. Questions 3. The batch process does not include any heat integration. I (i) List the various heat loads and outline a strategy to allow for the inclusion of a simple HEN with this single equipment unit. (ii) The overall objective of the HEN would be to reduce the total utility costs (the total amount of heating and cooling required) by 50%. Do you think this is an attainable ambition? (iii) Why are HENs much more challenging to design with just a single equipment unit Process review 1 (PR1): this is carried out with the objective of increasing the production rate of product P (per 24 hours) with the current equipment set-up. Comment on the following three options: 4a. As things currently stand (situation on page 1), the reactor conversion is 50%. It is suggested that the reactor conversion might be increased to 90% to increase the value of (nP)end,, or perhaps reduced to 20% to reduce the value of (nP)end. Describe how to determine the optimum X value to maximise the throughput of P. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500L, as stated initially. 4b. The throughput might also be increased by running at a higher temperature, say 80C. At this temperature, k80 is double the value at k50. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500L, as stated initially. 4c. The volume and initial amounts of the two reactants might be varied from the initial values of 500L,120 moles and 120 moles respectively. The maximum solubilities for A and B in ethanol at 50C are given on the previous page. At 80C, it can be assumed that the maximum solubilities for A and B are both twice the values at 50C. Hint: You are advised to write down the equation for the throughput per 24 hours, but using variables for the terms that are allowed to vary rather than the numbers as per the above. Assignment 1: Design of batch processes Process description A batch process is operated with the following sequence. The feed to the batch process consists of a solution of 500L of ethanol with 120 mole of A and 120 mole of B. If the temperature of the solution is sufficient, A and B will react to form P (the desired product): A(eth)+B(eth)P(s)Hreact=100kJ/mol Note that the product P is sparingly soluble in ethanol, which helps facilitate its removal from the reaction solution. It can be assumed that the reaction rate is first-order in both the concentration of A(cA) and B(CB), and so second-order overall: rA=kcAcB The batch process consists of the following FOUR steps: Initially, the tank is empty. 1. Fill tank with solution (ethanol/A/B). Increase temperature of the feed from 20C to 50C (1 hour) 2. Reaction takes place isothermally at 50C (4 hours, to give conversion of 50% ) 3. Filtration of reaction solution to give product P ( 2 hours) 4. Drying with hot nitrogen gas followed by the removal of powder product ( 3 hours) 6. The filtrate (containing the unreacted A and B ) is then returned to the reaction vessel, where the components are topped up to the initial amounts specified above. The time for the transfer and top-up can be neglected. The whole process is then repeated. CP974: Assignment 1(2022/23) Information on stages 1. It can be assumed that the heating time for Stage 1(t1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (t2) is related to the fractional conversion (X) by: Information on stages 1. It can be assumed that the heating time for Stage 1(t1) is proportional to the change in temperature (which is 30C in the case above). This would assume a constant-power heater. The heat capacity of the solution can be taken to be 500kJ/K. 2. For a batch reactor, assuming an overall second-order dependency, the reaction time (t2) is related to the fractional conversion (X) by: t2=k(cA)01(1XX) where k is the rate constant (which increases with temperature) and (cA)0 is the initial concentration of component A. At the completion of the reaction, the final concentration and amount of product are (cP)end and (nP)end respectively. 3. For batch filtration, the filtration time (t3) is defined as the time taken for the entire solvent to pass through the filter case, which will increase with volume of filtrate. As the filter cake increases in depth, so does the resistance to flow which will give a reduction in volumetric flowrate. From Darcy's law and constant P filtration, it can be shown that: t3(np)endVend The final filtrate ( Vend ) is assumed to be 500L for the above case. As an example, if the volume is doubled, keeping the concentration the same, the filtration time will be quadrupled. 4. Because an excess amount of hot gas is used - the product should be bone dry for quality control purposes - the drying time (t4) can be considered to be independent of the final cake thickness, and the large value is for product quality reasons. 5. For ethanol, the maximum solubility values are 0.6mol/L and 0.25mol/L for components A and B respectively at 50C. These are the maximum amounts that will dissolve in the solvent. If more A and B is added, this will remain in the solid phase. Questions 3. The batch process does not include any heat integration. I (i) List the various heat loads and outline a strategy to allow for the inclusion of a simple HEN with this single equipment unit. (ii) The overall objective of the HEN would be to reduce the total utility costs (the total amount of heating and cooling required) by 50%. Do you think this is an attainable ambition? (iii) Why are HENs much more challenging to design with just a single equipment unit Process review 1 (PR1): this is carried out with the objective of increasing the production rate of product P (per 24 hours) with the current equipment set-up. Comment on the following three options: 4a. As things currently stand (situation on page 1), the reactor conversion is 50%. It is suggested that the reactor conversion might be increased to 90% to increase the value of (nP)end,, or perhaps reduced to 20% to reduce the value of (nP)end. Describe how to determine the optimum X value to maximise the throughput of P. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500L, as stated initially. 4b. The throughput might also be increased by running at a higher temperature, say 80C. At this temperature, k80 is double the value at k50. Hint: You are advised to use a programme such as Excel to obtain a graph of throughput against X. You should assume the initial volume of ethanol is 500L, as stated initially. 4c. The volume and initial amounts of the two reactants might be varied from the initial values of 500L,120 moles and 120 moles respectively. The maximum solubilities for A and B in ethanol at 50C are given on the previous page. At 80C, it can be assumed that the maximum solubilities for A and B are both twice the values at 50C. Hint: You are advised to write down the equation for the throughput per 24 hours, but using variables for the terms that are allowed to vary rather than the numbers as per the above










