Question
At 20C, the hydrolysis of substance A in aqueous solution was carried out, starting of a concentration of 0.05 moles of A per liter.
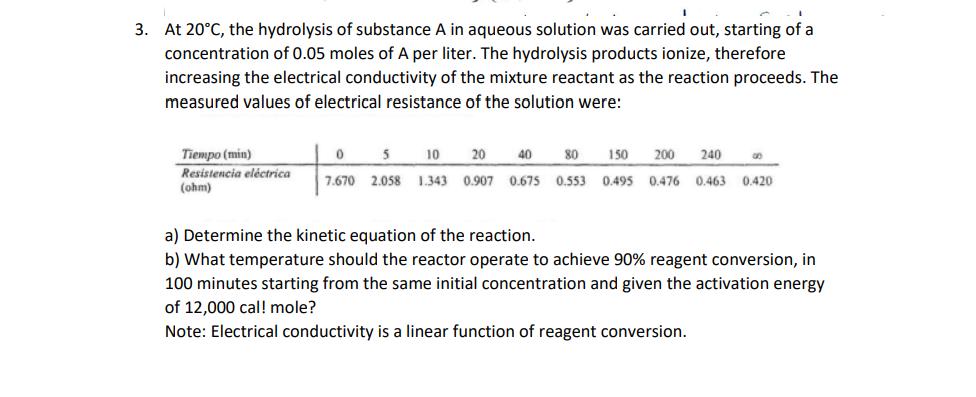
At 20C, the hydrolysis of substance A in aqueous solution was carried out, starting of a concentration of 0.05 moles of A per liter. The hydrolysis products ionize, therefore increasing the electrical conductivity of the mixture reactant as the reaction proceeds. The measured values of electrical resistance of the solution were: Tiempo (min) Resistencia elctrica (ohm) 5 10 20 40 80 150 200 240 7.670 2.058 1.343 0.907 0.675 0.553 0.495 0.476 0.463 0.420 0 a) Determine the kinetic equation of the reaction. b) What temperature should the reactor operate to achieve 90% reagent conversion, in 100 minutes starting from the same initial concentration and given the activation energy of 12,000 cal! mole? Note: Electrical conductivity is a linear function of reagent conversion.
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Algebra Graphs and Models
Authors: Marvin L. Bittinger, Judith A. Beecher, David J. Ellenbogen, Judith A. Penna
5th edition
321845404, 978-0321791009, 321791002, 978-0321783950, 321783956, 978-0321845405
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



