Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At 88.2 K, Ne occupies 12.74 liters at a pressure of 0.606 atm. Calculate the pressure, temperature and volume for Ar if it is
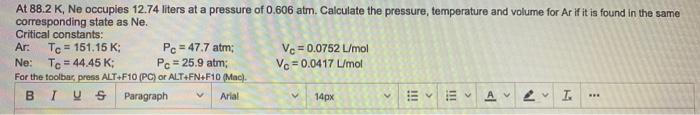
At 88.2 K, Ne occupies 12.74 liters at a pressure of 0.606 atm. Calculate the pressure, temperature and volume for Ar if it is found in the same corresponding state as Ne. Critical constants: Ar: Pc = 47.7 atm; Pc = 25.9 atm; Te = 151.15 K; Vc= 0.0752 L/mol Ve = 0.0417 L/mol Ne: Te = 44.45 K; For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arlal 14px ... I!! !!!
Step by Step Solution
★★★★★
3.34 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
Given data are 1 at any given state parameters of Ne are PressureP 0606 atm TemperatureT 882 K Volum...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
60d452b083bfd_227711.pdf
180 KBs PDF File
60d452b083bfd_227711.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


