Answered step by step
Verified Expert Solution
Question
1 Approved Answer
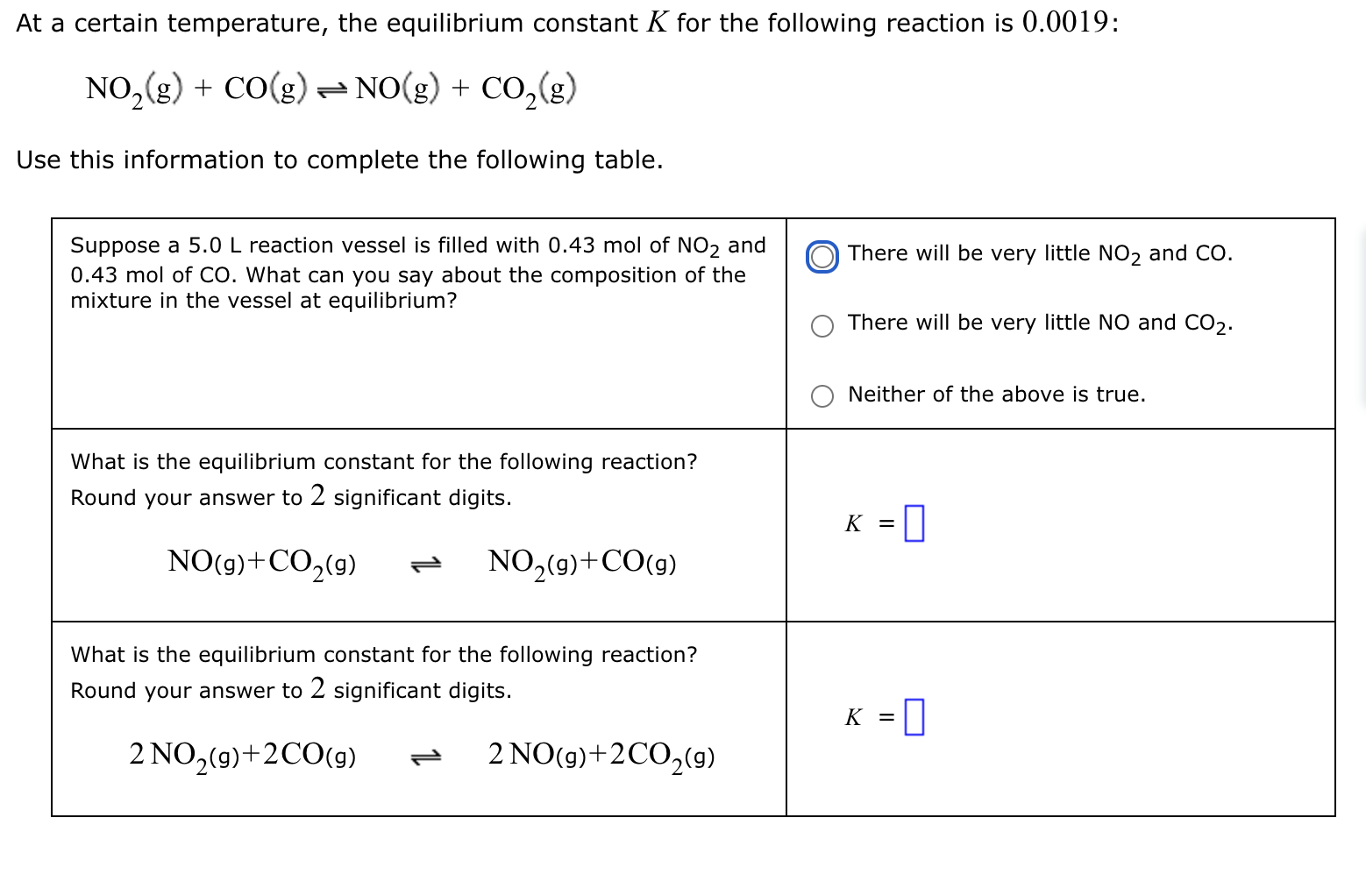
At a certain temperature, the equilibrium constant for the following reaction is : Use this information to complete the following table. Suppose a 5 .
At a certain temperature, the equilibrium constant for the following reaction is :
Use this information to complete the following table.
Suppose a L reaction vessel is filled with mol of NO and mol of CO What can you say about the composition of the mixture in the vessel at equilibrium?
There will be very little NO and CO
There will be very little NO and CO
Neither of the above is true.
What is the equilibrium constant for the following reaction? Round your answer to significant digits.
gggg
What is the equilibrium constant for the following reaction? Round your answer to significant digits.
gggg
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


