Question
At a certain temperature, the equilibrium partial pressures for the substances in the reaction 2 N205 (g) 02 (g) +4 NO2 (g) were: PN205=3.21
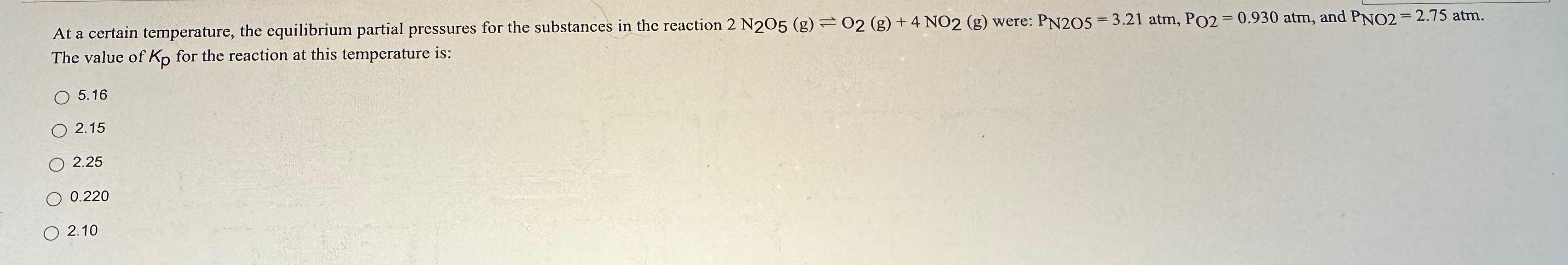
At a certain temperature, the equilibrium partial pressures for the substances in the reaction 2 N205 (g) 02 (g) +4 NO2 (g) were: PN205=3.21 atm, PO2 = 0.930 atm, and PNO2 = 2.75 atm. The value of Kp for the reaction at this temperature is: 5.16 2.15 2.25 0.220 2.10
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Markets And Institutions
Authors: Frederic S. Mishkin, Stanley G. Eakins
7th Edition
013213683X, 978-0132136839
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



