Question
At a certain temperature, the Kp for the decomposition of H2S is 0.755. HS(g) H2(g) + S(g) Initially, only H2S is present at a
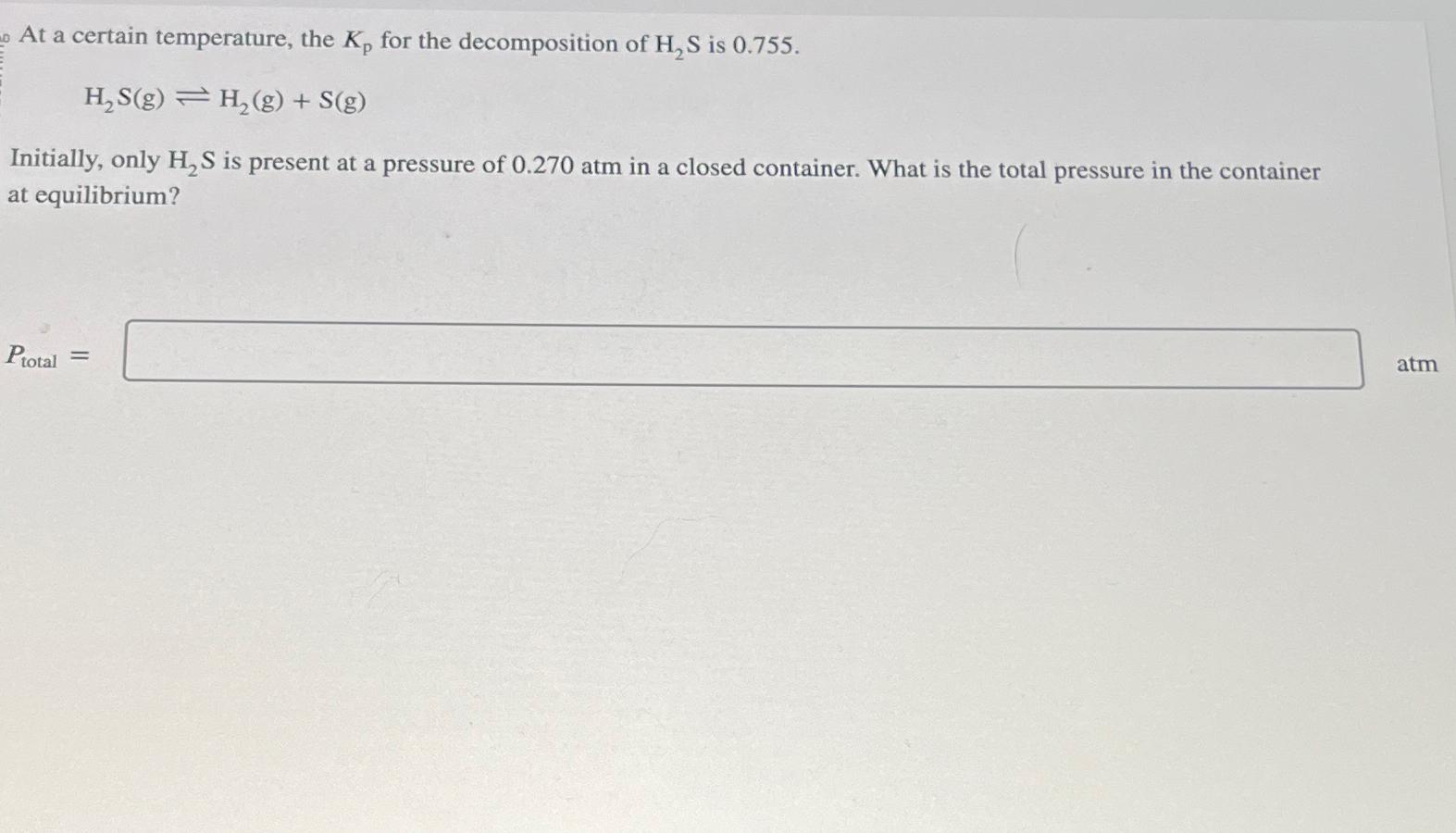
At a certain temperature, the Kp for the decomposition of H2S is 0.755. HS(g) H2(g) + S(g) Initially, only H2S is present at a pressure of 0.270 atm in a closed container. What is the total pressure in the container at equilibrium? Ptotal = atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To determine the total pressure in the container at equilibrium for the decomposition of HS we can u...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



