Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At a fixed temperature, equal moles of N2 (g) and H2 (g) are mixed in a constant pressure container (the volume of the container
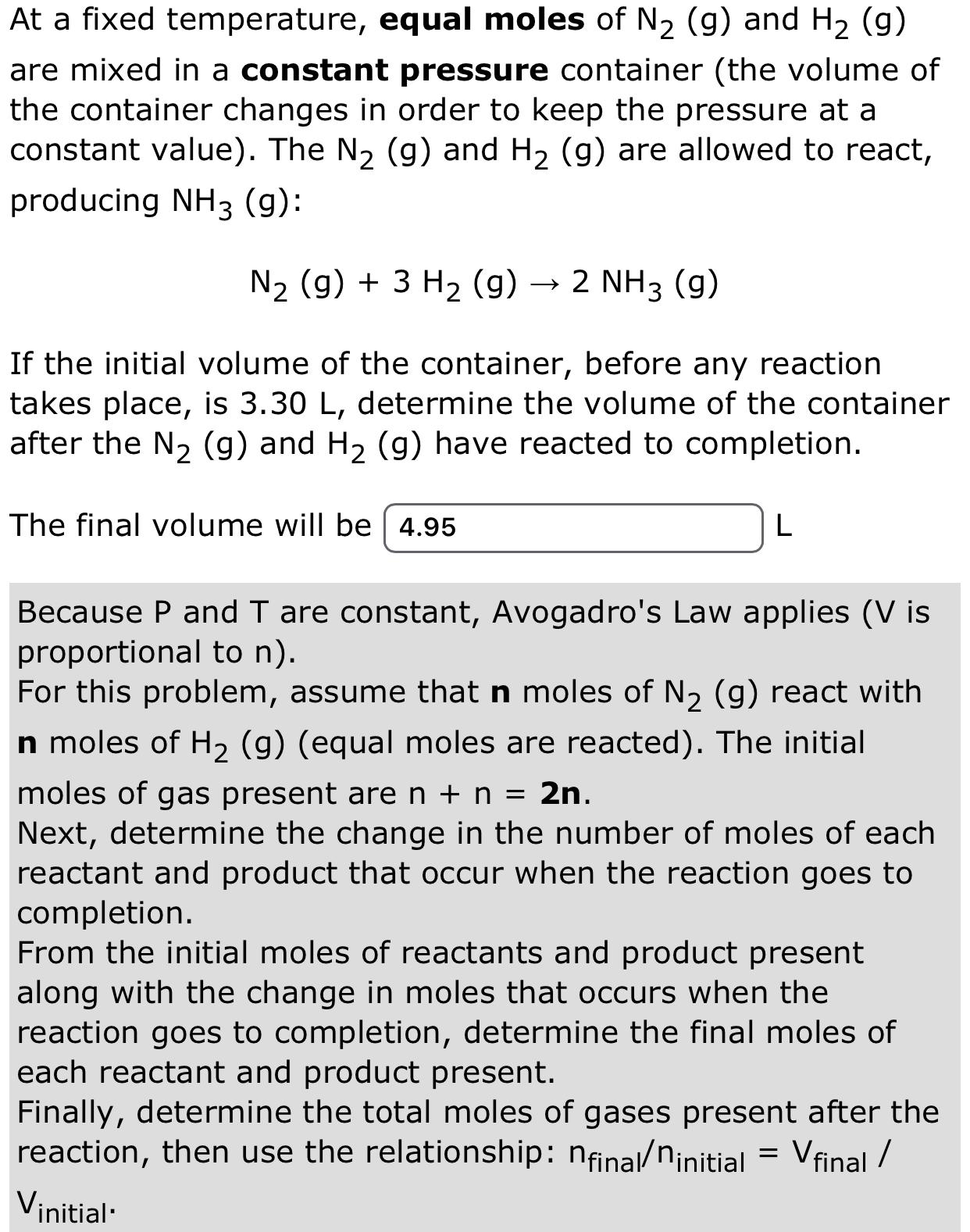
At a fixed temperature, equal moles of N2 (g) and H2 (g) are mixed in a constant pressure container (the volume of the container changes in order to keep the pressure at a constant value). The N2 (g) and H2 (g) are allowed to react, producing NH3 (9): N2 (g) + 3 H2 (g) - > 2 NH3 (9) If the initial volume of the container, before any reaction takes place, is 3.30 L, determine the volume of the container after the N2 (g) and H2 (g) have reacted to completion. The final volume will be 4.95 L Because P and T are constant, Avogadro's Law applies (V is proportional to n). For this problem, assume that n moles of N2 (g) react with n moles of H2 (g) (equal moles are reacted). The initial moles of gas present are n + n = 2n. Next, determine the change in the number of moles of each reactant and product that occur when the reaction goes to completion. From the initial moles of reactants and product present along with the change in moles that occurs when the reaction goes to completion, determine the final moles of each reactant and product present. Finally, determine the total moles of gases present after the reaction, then use the relationship: nfinal/ninitial = V final / Vinitial'
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Given the balanced chemical equation for the reaction and the initial con...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


