Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Given the following thermodynamic data for this reaction: H2PO4 (aq) + H2O (1) HPO4 (aq) + H3O+ (aq) H2PO4 (aq) AG (kJ/mol) -1130 a.
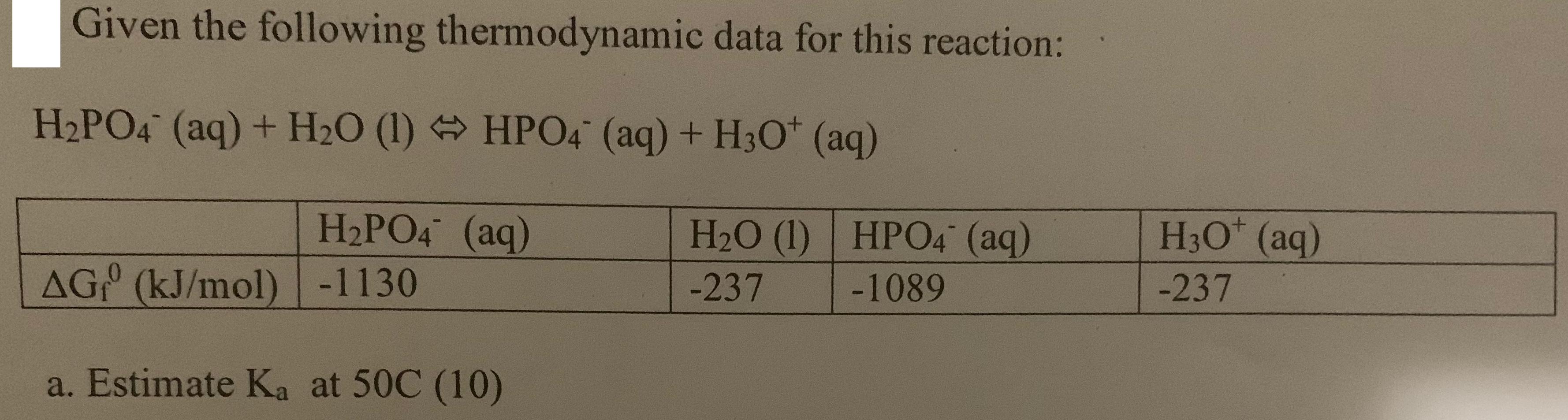
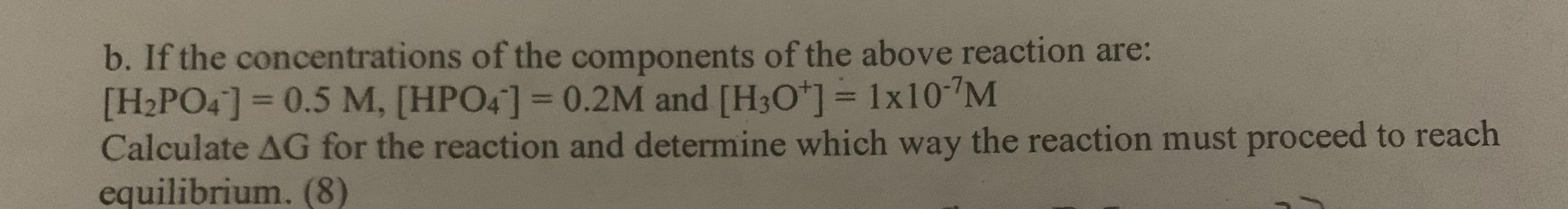
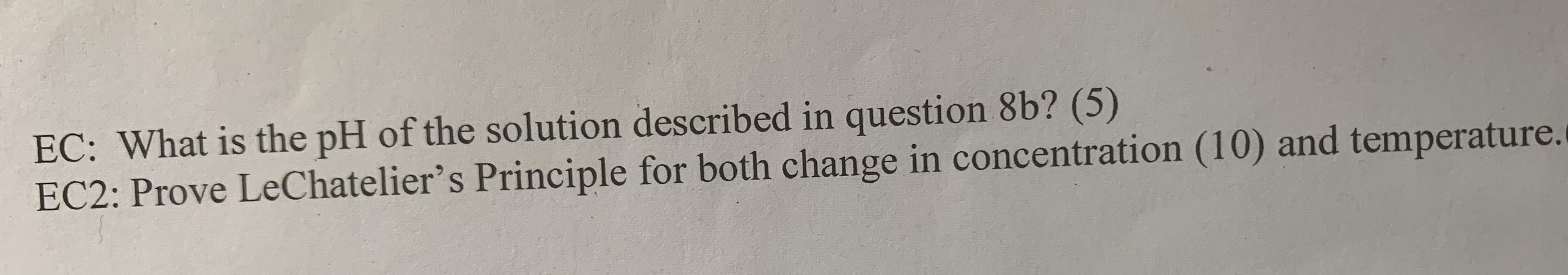
Given the following thermodynamic data for this reaction: H2PO4 (aq) + H2O (1) HPO4 (aq) + H3O+ (aq) H2PO4 (aq) AG (kJ/mol) -1130 a. Estimate Ka at 50C (10) H2O (1) HPO4 (aq) H3O+ (aq) -237 -1089 -237 b. If the concentrations of the components of the above reaction are: [H2PO4] = 0.5 M, [HPO4] = 0.2M and [H3O+] = 1x107M Calculate AG for the reaction and determine which way the reaction must proceed to reach equilibrium. (8) EC: What is the pH of the solution described in question 8b? (5) EC2: Prove LeChatelier's Principle for both change in concentration (10) and temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To estimate Ka at 50C 10C we can use the relationship between the standard free energy change G and the equilibrium constant K at a given temperature G RT lnK Where G standard free energy change R g...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


