Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. One form of Henderson-Hasselbalch Equation shown below ( derived from the generic equation for an acid-dissociation reaction. It is useful for approximating the
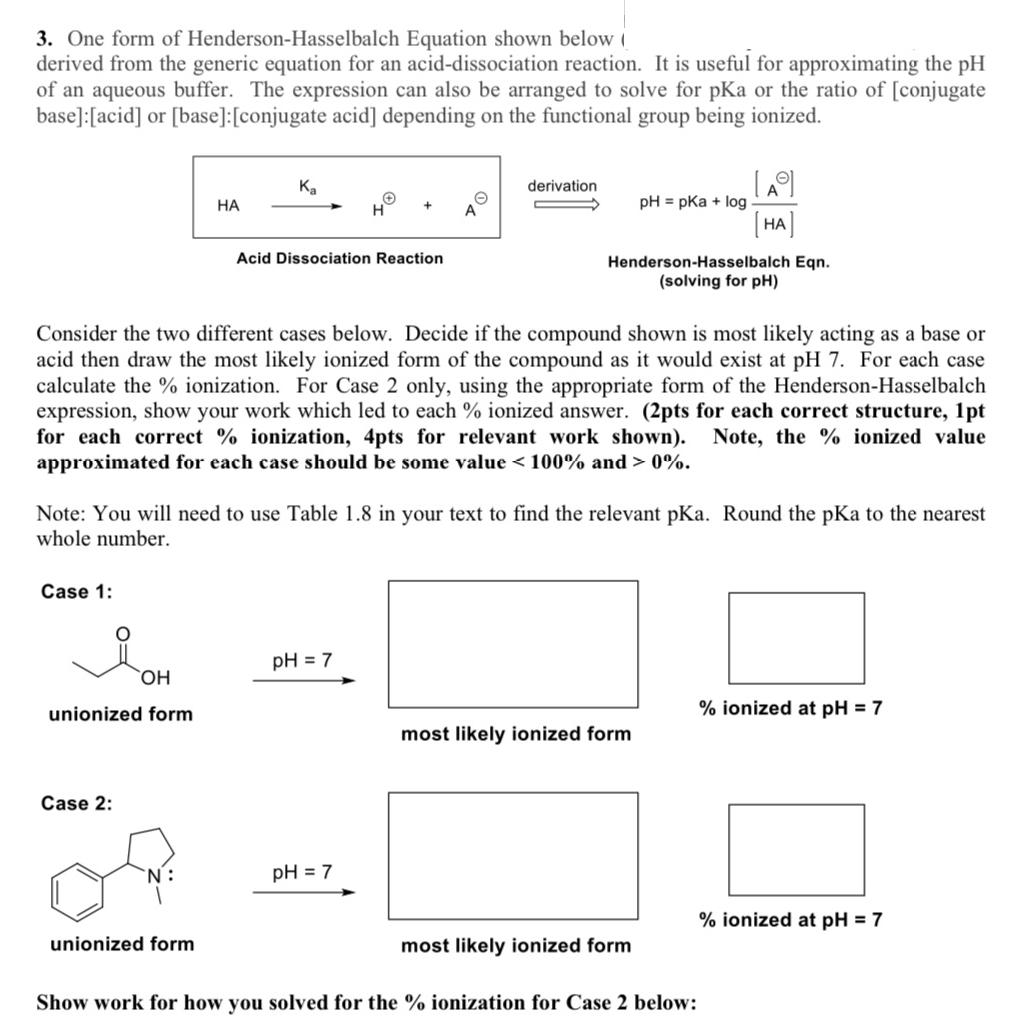
3. One form of Henderson-Hasselbalch Equation shown below ( derived from the generic equation for an acid-dissociation reaction. It is useful for approximating the pH of an aqueous buffer. The expression can also be arranged to solve for pKa or the ratio of [conjugate base]: [acid] or [base]: [conjugate acid] depending on the functional group being ionized. Ka derivation HA + pH = pKa + log H A [A] [HA] Acid Dissociation Reaction Henderson-Hasselbalch Eqn. (solving for pH) Consider the two different cases below. Decide if the compound shown is most likely acting as a base or acid then draw the most likely ionized form of the compound as it would exist at pH 7. For each case calculate the % ionization. For Case 2 only, using the appropriate form of the Henderson-Hasselbalch expression, show your work which led to each % ionized answer. (2pts for each correct structure, 1pt for each correct % ionization, 4pts for relevant work shown). Note, the % ionized value approximated for each case should be some value < 100% and > 0%. Note: You will need to use Table 1.8 in your text to find the relevant pKa. Round the pKa to the nearest whole number. Case 1: pH = 7 OH unionized form Case 2: unionized form pH = 7 % ionized at pH = 7 most likely ionized form % ionized at pH = 7 most likely ionized form Show work for how you solved for the % ionization for Case 2 below:
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Case 1 OH The compound is most likely acting as a base at pH ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


