Answered step by step
Verified Expert Solution
Question
1 Approved Answer
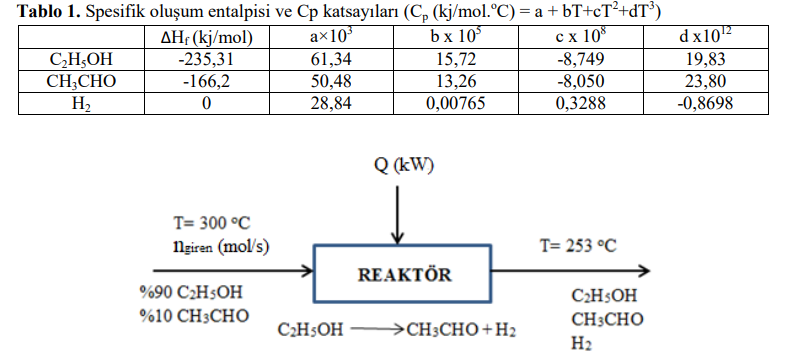
At the flow rate given in Table 1, 90% ethanol and 10% acetaldehyde at 300 oC demol is fed to the reactor where the ethanol
At the flow rate given in Table 1, 90% ethanol and 10% acetaldehyde at 300 oC demol is fed to the reactor where the ethanol dehydrogenation reaction takes place. The ethanol conversion in the reactor is 32%. Heat is supplied to the reactor to prevent the temperature from falling too low and therefore the reaction rate from falling to an unacceptably low level. If the reactor outlet temperature is 253 oC, calculate the amount of heat (Q (kW)) that should
Amount of feed entering the reactor (mol/s)=350
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


