Answered step by step
Verified Expert Solution
Question
1 Approved Answer
atm? mol? I keep getting wrong numbers. water Complete the fol 14 Inverteudiometer into beaker of water Verify your calculation in your set up. do
atm? mol? I keep getting wrong numbers. 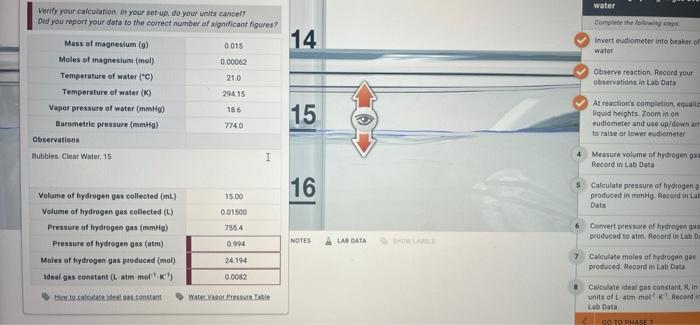
water Complete the fol 14 Inverteudiometer into beaker of water Verify your calculation in your set up. do your units cancel? Did you report your data to the correct number of significant igures? Mass of magnesium (a) 0015 Moles of magnesium (mol) 0.00062 Temperature of water (C) 21.0 Temperature of water (K) 29415 Vapor pressure of water (mmHg) 186 Barometrie pressure (mmHg) 7740 Observations Bubbles Clear Water 15 1 Observe reaction Record your observations lo Lab Data 15 At reaction's completion quali liquid helghts Zoom in on eudiometer and use up/down a to raise or lower esdiometer Measure volume of hydrogen gam Record in Lab Data 16 Calculate pressure of hydrogen produced in mmHg. Record in La Data 15.00 0.01500 7554 Volume of hydrogen ges collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mm) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L atm moll) Convert pressure of tydrogenas produced to atm. Record in Lab Da 0.94 NOTES LAS DATA 7 24.194 Calculate moles of hydrogen go produced. Record in Lab Data 0.0082 to setorant Water Calculate ideal gas constantin units of L-am mork Record Lab Data GO TO PHASE 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


