Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Atomic emission spectroscopy and the method of standard addition are used to determine the Na* concentration and its uncertainty in an unknown sample. Increasing
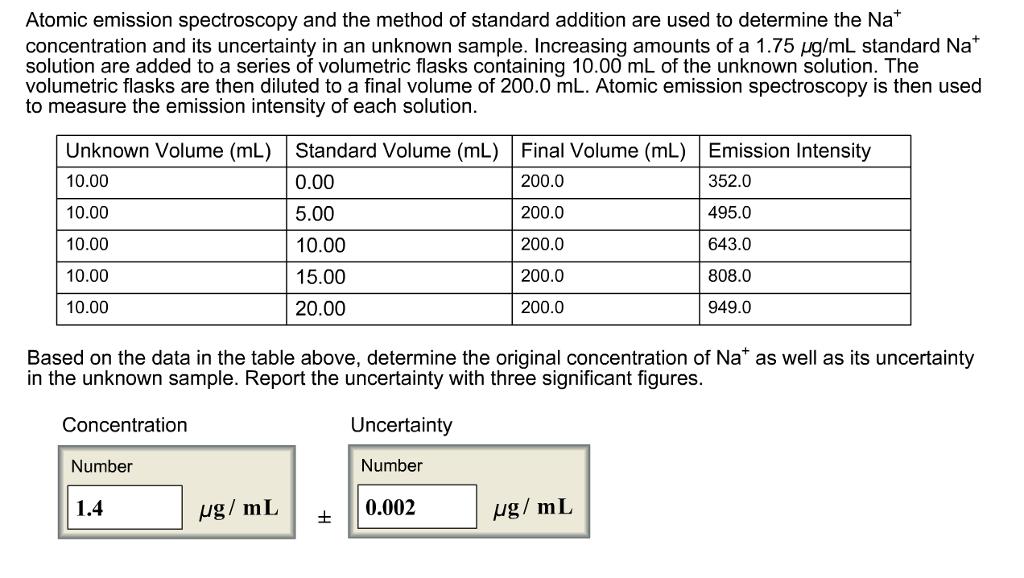
Atomic emission spectroscopy and the method of standard addition are used to determine the Na* concentration and its uncertainty in an unknown sample. Increasing amounts of a 1.75 ug/mL standard Na* solution are added to a series of volumetric flasks containing 10.00 mL of the unknown solution. The volumetric flasks are then diluted to a final volume of 200.0 mL. Atomic emission spectroscopy is then used to measure the emission intensity of each solution. Unknown Volume (mL) Standard Volume (mL) Final Volume (mL) Emission Intensity 10.00 0.00 200.0 352.0 10.00 5.00 200.0 495.0 10.00 10.00 200.0 643.0 10.00 15.00 200.0 808.0 10.00 20.00 200.0 949.0 Based on the data in the table above, determine the original concentration of Na* as well as its uncertainty in the unknown sample. Report the uncertainty with three significant figures. Concentration Uncertainty Number Number 1.4 g/ mL 0.002 ug/ mL
Step by Step Solution
★★★★★
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
From graph and spreadsh...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


