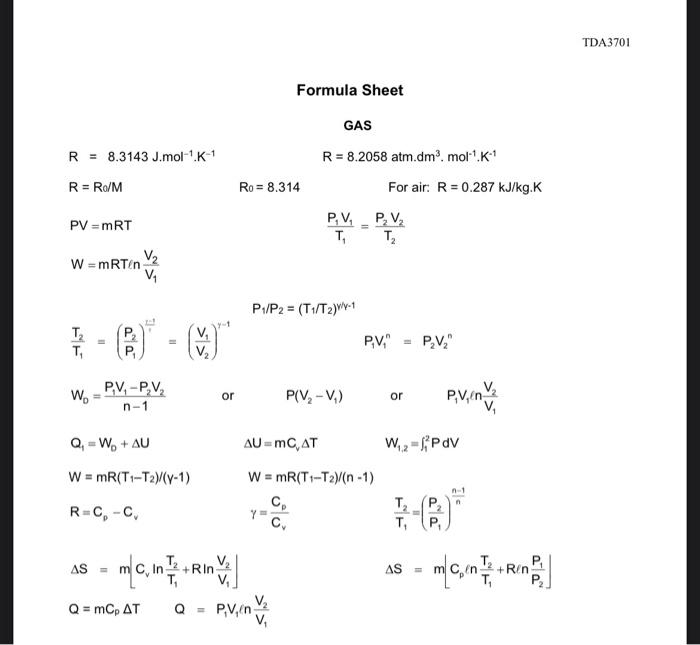
attached is the formula Sheet
Violia, a South Africa design, manufacture, testing and supply company has been asked to design an air vessel that can store air. A Chemical Engineering student from Unisa has been hired to test the vessel's capacity to withstand changes in temperature and pressure. The student has been given a vessel of capacity 12.3m3 which contains air at a pressure of 180kPa and a temperature of 42C. The vessel's pressure and temperature are reduced to 75kPa and 13C, respectively, by removing some air. The student needs to find the amount of air removed and the volume of this mass of air at the initial state of air. Take R=0.287kJ/kg.K for air. [12marks] QUESTION 2 A centrifugal compressor must compress a perfect gas with a molecular weight of 44. The perfect gas is compressed from 101.3kPa,27C to a pressure of 680kPa following the law PV1.2= constant. Take the specific heat at constant pressure of the gas as 0.846kJ/kg.K. Find the heat transfer and its direction. TDA 3701 Formula Sheet GAS R=8.3143Jmol1K1R=8.2058atmdm3mol1K1R=R/MR0=8.314Forair:R=0.287kJ/kg.KPV=mRTT1P1V1=T2P2V2W=mRTnV1V2P1/P2=(T1/T2)w/1T1T2=(P1P2)2=(V2V1)1P1V1n=P2V2nW0=n1P1V1P2V2orP(V2V1)orP1V1V1V2Q1=Wo+UU=mCrTW1,2=12PdVW=mR(T1T2)/(Y1)W=mR(T1T2)/(n1)R=CpCv=CvCpT1T2=(P1P2)nn1S=m[CvlnT1T2+RlnV1V2S=m[CpT1T2+RP2P1Q=mCpTQ=P1V1V1V2 TDA370! BOILERS 6=m1cvm1[h1h2]1100%Ec=mt2257mt[h1h0]hee=mj/mu(4.187)(t01t0)hr=mvmm[(4.187)(t4t01)+xh0]hs=m1mi(hsuehw)CONDENSERSms(xht1)+ms(4,187)(tkt2)=mmine(4,187)(t2t1)PaVa=MaRToPc=7,5BnVmPv=Pz+Pvm=V9V=V9Va s=tst1t2t1wec=BnPvVm100Q=UATimA=d0LN2V=4ds2NtCmw=4d12N2CTim=inTst1Tst2(Tst2)(Tstt) RANKINE CYCLE W=h1h2n=h1h3h1h2100%W=n(P1V1P2V2)(n1)MT=M,(h1h0)BP100 r=kar SSC=w3600 CARNOT CYCLE R=CpCv n=1T1T2 r=V4V1=P1P4=V3V2=P2P3S=mRinr NOZZLES mCv=VAC=44,72h1he=44,72Cp(TiTc)Ve=xVvx=hshch1x=sssiss1 P1PtmVsue=(+12)rt1=Vs1C1A1=P10,23(hsup1942) iv [TURN OVER] FLOW PROCESSES 2000V12+h1+Q12=2000V22+h2+W12 h=CpT m=VsvAQ=mCT REFRIGERATION COP=WQ C1=T2T1T1CA=IDQAQA=m1{4,187(tm0)+Li}CT=WTQT NON-FLOW PROCESSES h=PV+UQ=W0+U STEAM Ce=4.187kJkg1K1hg=hf+hiahw=hf+xh15hmp=hg+cp(tet)x=V7V2V2=Vtive=P20.23(h21942) JOULE CYCLE 0=1[rp1]711rp=P1P2=P4P3T2=Trp1Wa=W1Wcrn=wn/w1Wa=mce(T2T4)mcp(T2T1)rs=cicbOTTOCYCLErv=V2V1=V2V1+V2Vs=4d2L=1(T4Ti)r01T4T1 COMPRESSORS v=1VsVc[(P1P2)n11]w=mRT1(n1n)[(P1P2)nn11]wi=P1V1n(P1P2) Quantities, symbols and units used: ce= specifie heat at constant pressure in kl/kg. K cy " specific heat at constant volame in kJ/kg.K. h= specific enthalpy in kl/kg p= absolute pressure in kPa s - specific entropy in k.I/kg.K T= temperature in C u= specifie internal energy k.1/kg TABLE OF PROPERTIES OF SATURATED WATER AND STEAM AS A FUNCTION OF PRESSURE TDA3701 TDA3701 PROPERTIES OF SATURATED WATER AND STEAM AS A FUNCTION OF TFMPFRATIIRF TABLE OF PROPERTIES OF SUPERHEATED STEAM TDA3701 TDA 3701 TDA3701 Saturated refrigerant 1248- Tengerature table x [TURN OVER] TDA3701 TDA3701 Saturated refrigerant- 134 - Piessure table xii [IURN OVER] TDA3701 Superheated refrigerant-134a (Continued)