Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the liquid alum (aluminum sulfate) volume to precipitate phosphorus from a wastewater containing 6 mg/L TP. The following data describe data for the
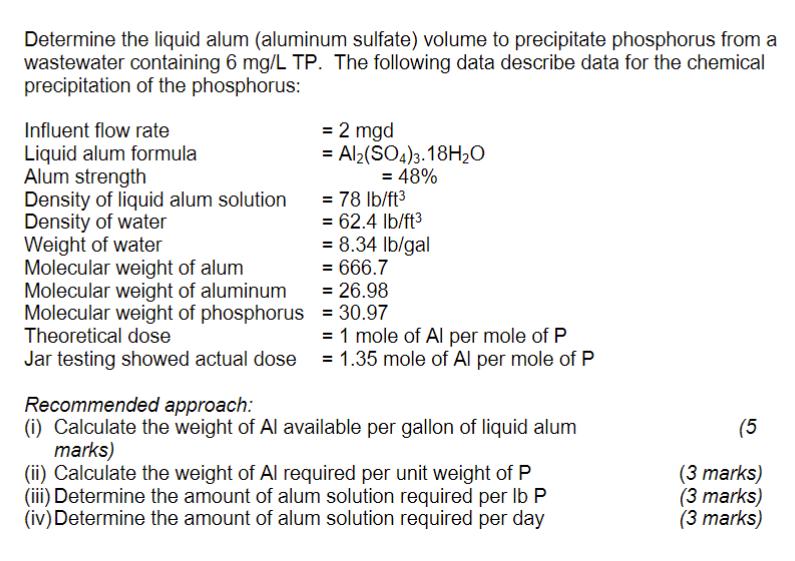
Determine the liquid alum (aluminum sulfate) volume to precipitate phosphorus from a wastewater containing 6 mg/L TP. The following data describe data for the chemical precipitation of the phosphorus: Influent flow rate Liquid alum formula Alum strength Density of liquid alum solution Density of water Weight of water Molecular weight of alum Molecular weight of aluminum Molecular weight of phosphorus Theoretical dose Jar testing showed actual dose = 2 mgd = Al2(SO4)3.18HO = 48% = 78 lb/ft = 62.4 lb/ft = 8.34 lb/gal = 666.7 = 26.98 = 30.97 = 1 mole of Al per mole of P = 1.35 mole of Al per mole of P Recommended approach: (i) Calculate the weight of Al available per gallon of liquid alum marks) (ii) Calculate the weight of Al required per unit weight of P (iii) Determine the amount of alum solution required per lb P (iv) Determine the amount of alum solution required per day (5 (3 marks) (3 marks) (3 marks)
Step by Step Solution
★★★★★
3.31 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
To determine the liquid alum volume required to precipitate phosphorus from the wastewater we can fo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


