Answered step by step
Verified Expert Solution
Question
1 Approved Answer
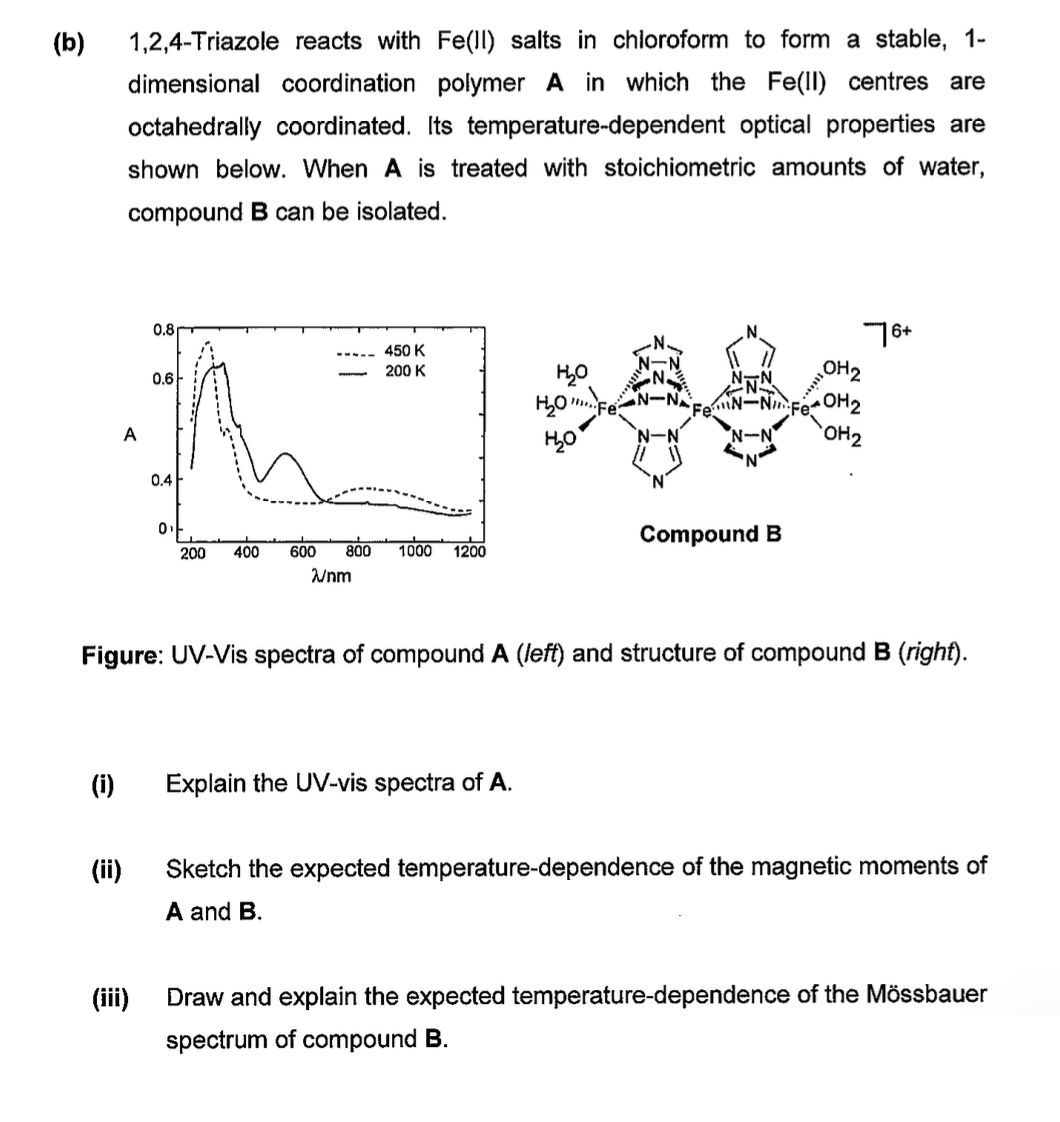
( b ) 1 , 2 , 4 - Triazole reacts with F e ( I l ) salts in chloroform to form a stable,
bTriazole reacts with salts in chloroform to form a stable,
dimensional coordination polymer in which the centres are
octahedrally coordinated. Its temperaturedependent optical properties are
shown below. When is treated with stoichiometric amounts of water,
compound can be isolated.
Figure: UVVis spectra of compound A left and structure of compound B right
i Explain the UVvis spectra of
ii Sketch the expected temperaturedependence of the magnetic moments of
A and
iii Draw and explain the expected temperaturedependence of the Mssbauer
spectrum of compound
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


