Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) Air at 5000kPa and 300K is flowing through a pipeline. An evacuated and insulated cylinder of volume 0.1m3 is connected to the pipeline through
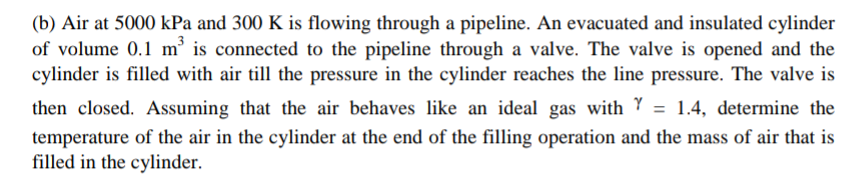
(b) Air at 5000kPa and 300K is flowing through a pipeline. An evacuated and insulated cylinder of volume 0.1m3 is connected to the pipeline through a valve. The valve is opened and the cylinder is filled with air till the pressure in the cylinder reaches the line pressure. The valve is then closed. Assuming that the air behaves like an ideal gas with =1.4, determine the temperature of the air in the cylinder at the end of the filling operation and the mass of air that is filled in the cylinder
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


