Question
(b) Calculate the concentrations of reactant and product for time intervals of 10 minutes for the reaction given in (4.1) and hence plot graphs
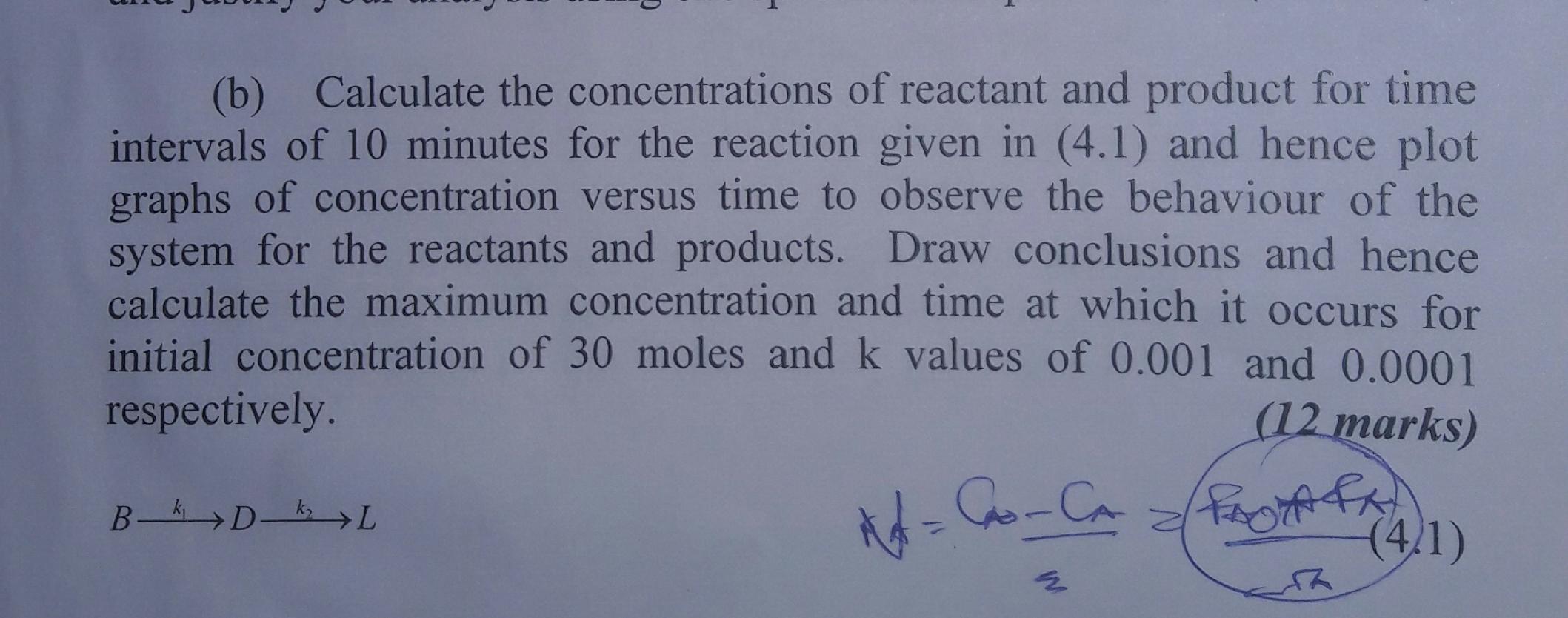
(b) Calculate the concentrations of reactant and product for time intervals of 10 minutes for the reaction given in (4.1) and hence plot graphs of concentration versus time to observe the behaviour of the system for the reactants and products. Draw conclusions and hence calculate the maximum concentration and time at which it occurs for initial concentration of 30 moles and k values of 0.001 and 0.0001 respectively. (12 marks) B- D- L (4.1)
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Date Paga B R 0001 min 31 1 Ra 0 00ol min a conaeq...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



