Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b- Calculate the standard enthalpy and entropy of the reaction in the interval 300K to 1100K. Can they be considered temperature independent? (hint: plotting the
b- Calculate the standard enthalpy and entropy of the reaction in the interval 300K to 1100K. Can they be considered temperature independent? (hint: plotting the results as a function of temperature could be instructive)
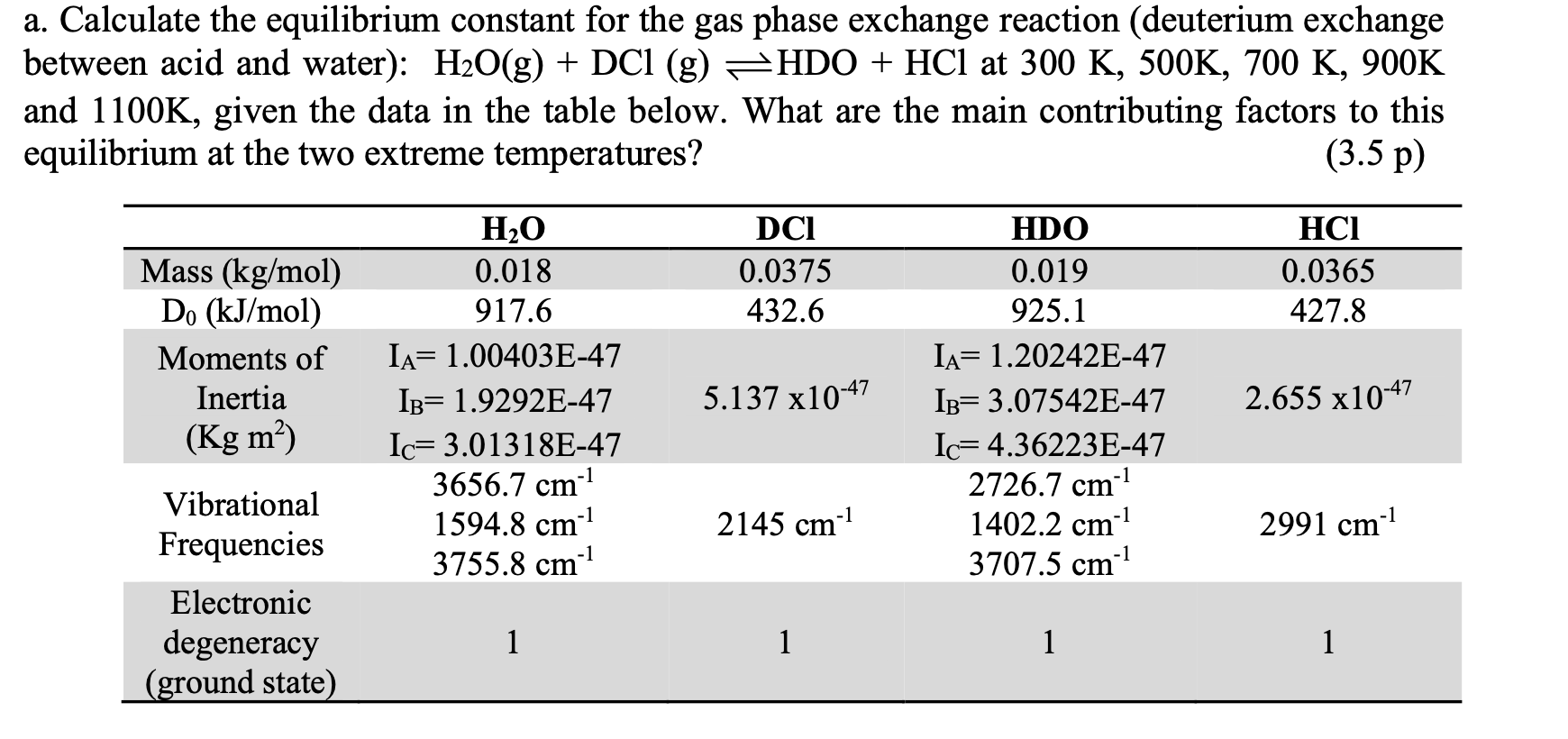
Calculate the equilibrium constant for the gas phase exchange reaction (deuterium exchange etween acid and water ):H2O(g)+DCl(g)HDO+HCl at 300K,500K,700K,900K nd 1100K, given the data in the table below. What are the main contributing factors to this quilibrium at the two extreme temperatures? (3.5p)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


