Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) Consider the liquid phase reaction cis-trans isomerization of 2-butene The reaction is first order and is carried out in a tubular reactor in which
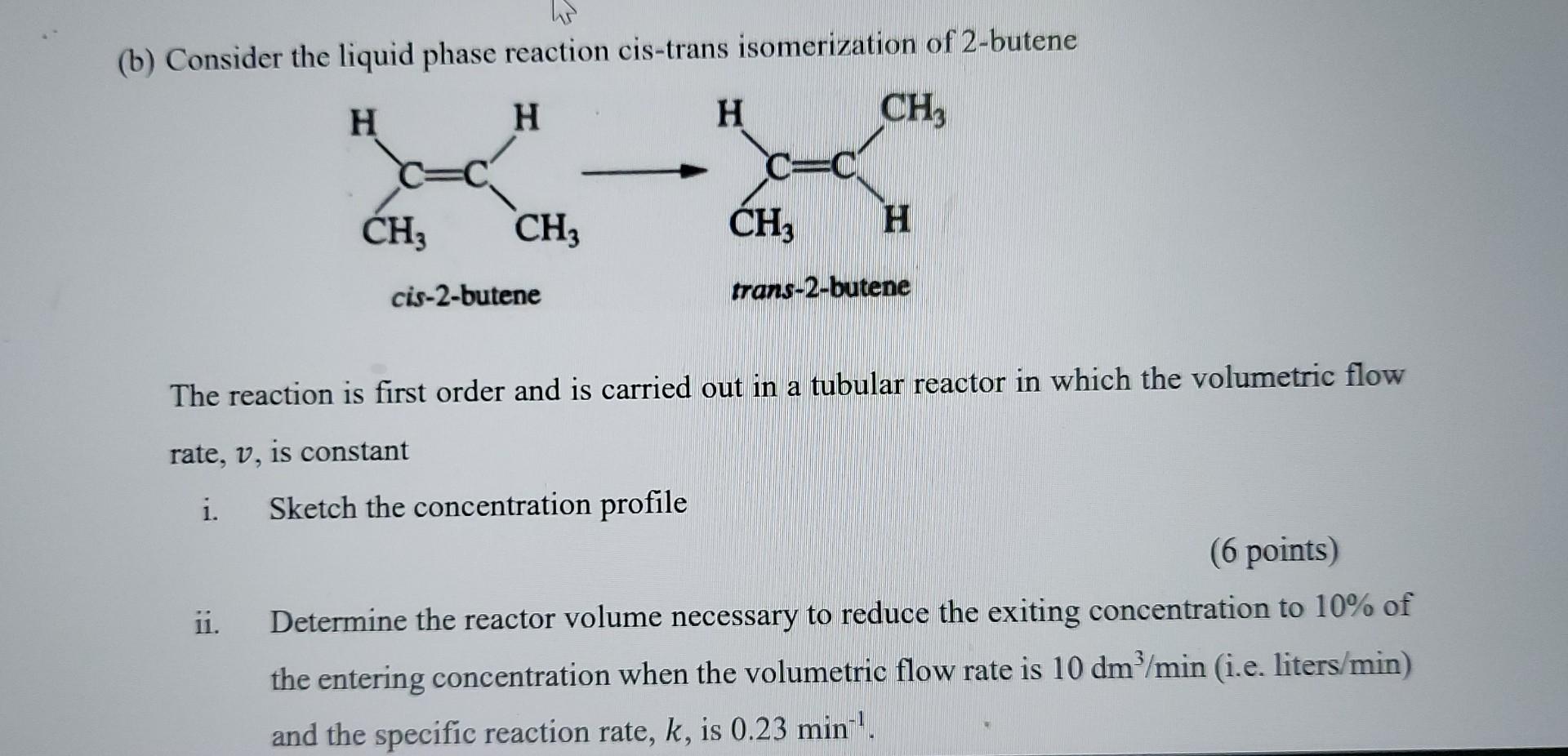
(b) Consider the liquid phase reaction cis-trans isomerization of 2-butene The reaction is first order and is carried out in a tubular reactor in which the volumetric flow rate, v, is constant i. Sketch the concentration profile (6 points) ii. Determine the reactor volume necessary to reduce the exiting concentration to 10% of the entering concentration when the volumetric flow rate is 10dm3/min (i.e. liters /min ) and the specific reaction rate, k, is 0.23min1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


