Question
b) Given the following reactions draw a p-pH diagram for aluminium. Assume a concentration of 10-6 M for Alt. For purposes of drawing the
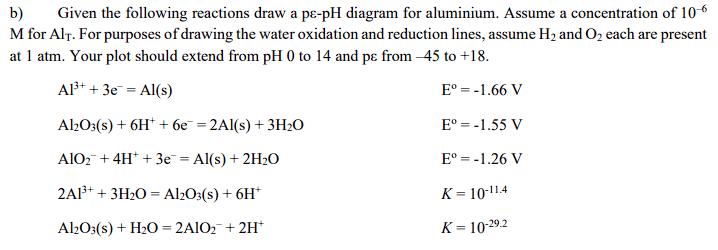
b) Given the following reactions draw a p-pH diagram for aluminium. Assume a concentration of 10-6 M for Alt. For purposes of drawing the water oxidation and reduction lines, assume H2 and O2 each are present at 1 atm. Your plot should extend from pH 0 to 14 and p from -45 to +18. Al3++3eAl(s) = Al2O3(s)+6H6e2Al(s) + 3H2O = AlO2+4H+3e Al(s) + 2H2O = 2A13+ 3H2O Al2O3(s) + 6H+ Al2O3(s) + H2O=2A102 + 2H+ E = -1.66 V E -1.55 V E = -1.26 V K-10-11.4 K-10-29.2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



