Question
B. How does this compare to the solubility of Mg(OH)2 in pure water? (S1S/S1S=?) Calculate the solubility (in grams per 1.00 x 10 mL of
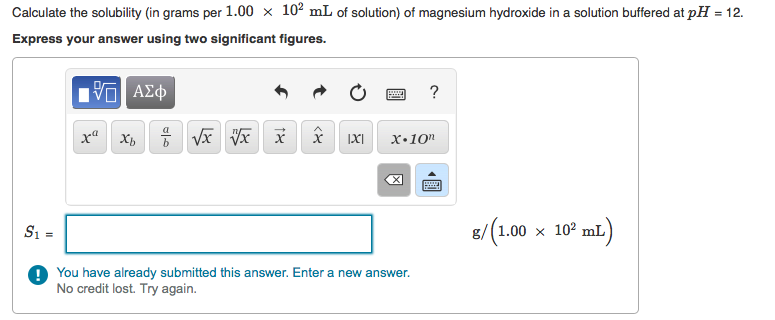
B. How does this compare to the solubility of Mg(OH)2 in pure water? (S1S/S1S=?)
Calculate the solubility (in grams per 1.00 x 10 mL of solution) of magnesium hydroxide in a solution buffered at pH = 12. Express your answer using two significant figures. IVE x Xb x x x IXI ? X.10n S = ! You have already submitted this answer. Enter a new answer. No credit lost. Try again. g/(1.00 10 mL)
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
As Ksp value of MgOH2 is not provided in your question I took it from external source due to its imp...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



