Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) (i) Describe preliminary analyses that can be carried out on crystals before using single crystal X-ray crystallography analysis. 3 (c) (i) The complex
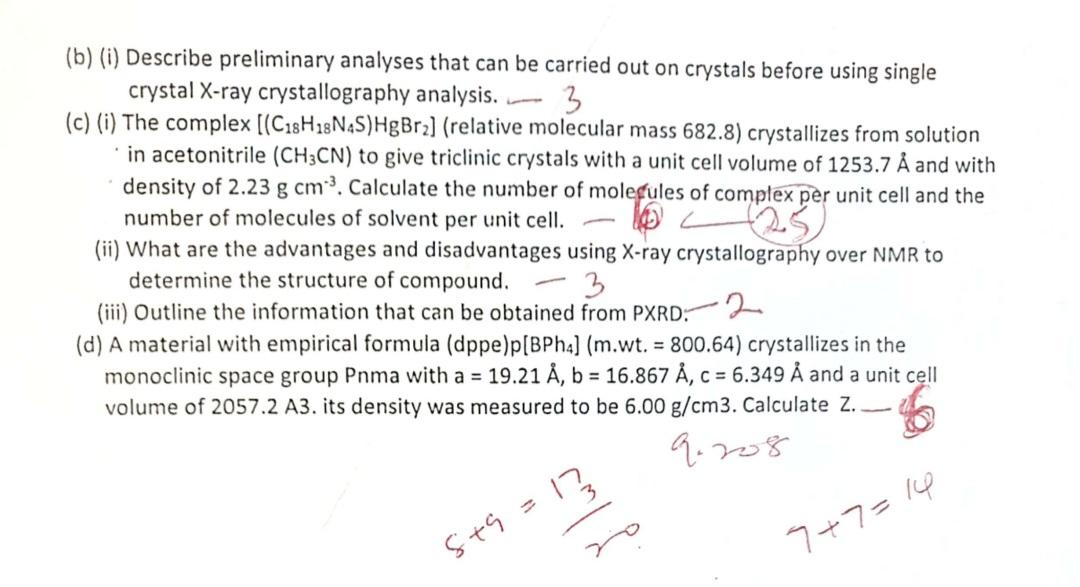
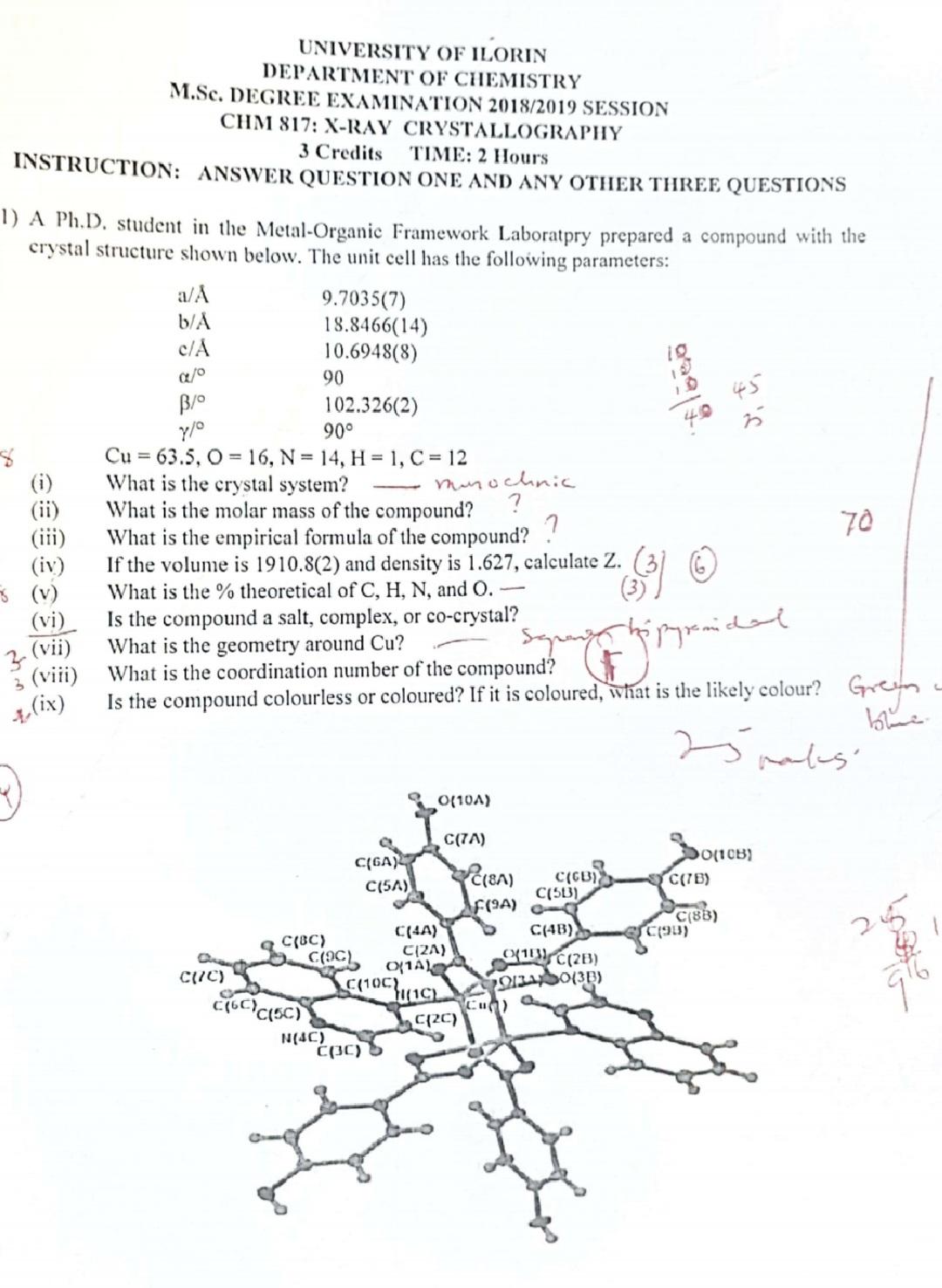
(b) (i) Describe preliminary analyses that can be carried out on crystals before using single crystal X-ray crystallography analysis. 3 (c) (i) The complex [(C18H18N4S)HgBr2] (relative molecular mass 682.8) crystallizes from solution in acetonitrile (CH3CN) to give triclinic crystals with a unit cell volume of 1253.7 and with density of 2.23 g cm. Calculate the number of molecules of complex per unit cell and the number of molecules of solvent per unit cell. - 25 (ii) What are the advantages and disadvantages using X-ray crystallography over NMR to determine the structure of compound. 3 (iii) Outline the information that can be obtained from PXRD 2 (d) A material with empirical formula (dppe) p[BPh4] (m.wt. = 800.64) crystallizes in the monoclinic space group Pnma with a = 19.21 , b = 16.867 , c = 6.349 and a unit cell volume of 2057.2 A3. its density was measured to be 6.00 g/cm3. Calculate Z.. 8+9= 9.208 7+7=14 UNIVERSITY OF ILORIN DEPARTMENT OF CHEMISTRY M.Sc. DEGREE EXAMINATION 2018/2019 SESSION CHM 817: X-RAY CRYSTALLOGRAPHY 3 Credits TIME: 2 Hours INSTRUCTION: ANSWER QUESTION ONE AND ANY OTHER THREE QUESTIONS 1) A Ph.D. student in the Metal-Organic Framework Laboratory prepared a compound with the crystal structure shown below. The unit cell has the following parameters: a/A b/ c/A o / 7/0 9.7035(7) 18.8466(14) 10.6948(8) 90 102.326(2) 90 19 (ii) (iii) (iv) (v) (vi) 3(vii) 3 (viii) (ix) = Cu 63.5, O 16, N = 14, H = 1, C=12 What is the crystal system? What is the molar mass of the compound? munochnic ? What is the empirical formula of the compound? If the volume is 1910.8(2) and density is 1.627, calculate Z. What is the % theoretical of C, H, N, and O. Is the compound a salt, complex, or co-crystal? What is the geometry around Cu? What is the coordination number of the compound? Is the compound colourless or coloured? If it is coloured, what is the likely colour? 70 Greyn blue 25 pales O(10A) C(7A) C(GA) C(5A) 0(10B) C(8A) C(GB) C(51) C(7B) F(9A) - C(BB) C(4A) C(4B) (98) C(BC) C(2A) Cac O1A 01C(2B) C(7C) C(10C) 91340(3B) (1C) C16C)C(5) C2C) N(4C) C(30)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


