Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) Iron is an important component of haemoglobin, the protein that transports oxygen around the body. Persons who are anaemic can take iron supplements
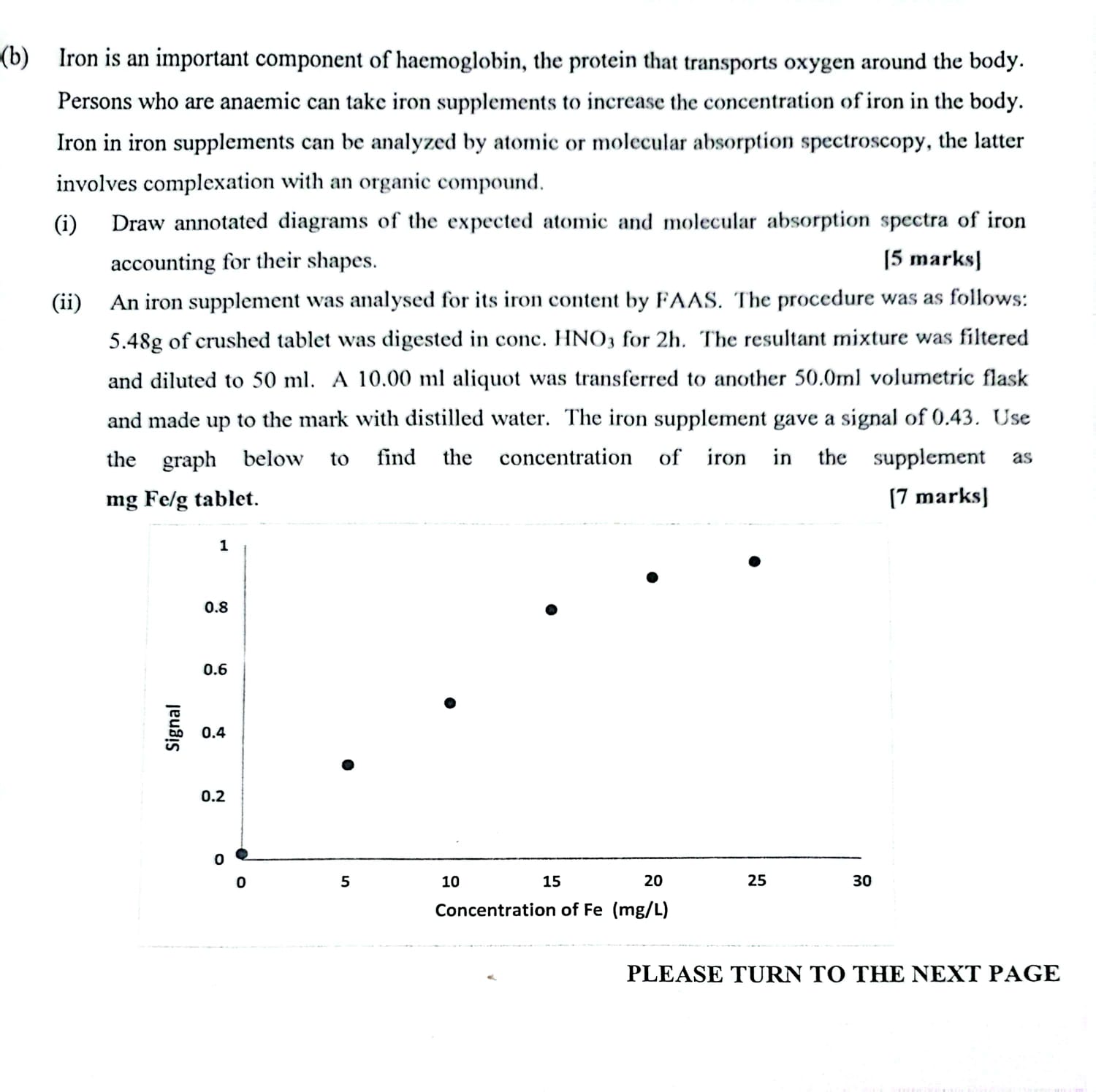
(b) Iron is an important component of haemoglobin, the protein that transports oxygen around the body. Persons who are anaemic can take iron supplements to increase the concentration of iron in the body. Iron in iron supplements can be analyzed by atomic or molecular absorption spectroscopy, the latter involves complexation with an organic compound. (i) Draw annotated diagrams of the expected atomic and molecular absorption spectra of iron accounting for their shapes. [5 marks] (ii) An iron supplement was analysed for its iron content by FAAS. The procedure was as follows: 5.48g of crushed tablet was digested in conc. HNO3 for 2h. The resultant mixture was filtered and diluted to 50 ml. A 10.00 ml aliquot was transferred to another 50.0ml volumetric flask and made up to the mark with distilled water. The iron supplement gave a signal of 0.43. Use the graph below to find the concentration of iron in the supplement as mg Felg tablet. [7 marks] Signal 1 0.8 0.6 0.4 0.2 5 20 15 Concentration of Fe (mg/L) 10 25 30 PLEASE TURN TO THE NEXT PAGE
Step by Step Solution
★★★★★
3.29 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Solution i Atomic and molecular absorption spectra ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


