Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) The first-order gas-phase isomerization reaction A * B with k = 5 min k5 ! is to be carried out in a tubular reactor.
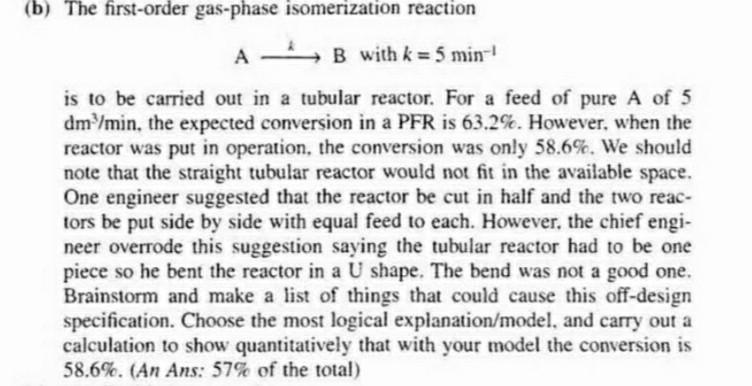
(b) The first-order gas-phase isomerization reaction A * B with k = 5 min k5 ! is to be carried out in a tubular reactor. For a feed of pure A of 5 dm /min, the expected conversion in a PFR is 63.2%. However, when the reactor was put in operation, the conversion was only 58.6%. We should note that the straight tubular reactor would not fit in the available space. One engineer suggested that the reactor be cut in half and the two reac- tors be put side by side with equal feed to each. However, the chief engi- neer overrode this suggestion saying the tubular reactor had to be one piece so he bent the reactor in a U shape. The bend was not a good one. Brainstorm and make a list of things that could cause this off-design specification. Choose the most logical explanation/model, and carry out a calculation to show quantitatively that with your model the conversion is 58.6%. (An Ans: 57% of the total)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


