Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) To protect an iron (Fe) rod of radius of 20 cm from being oxidized, a layer of chromium (Cr) is coated. Suppose the
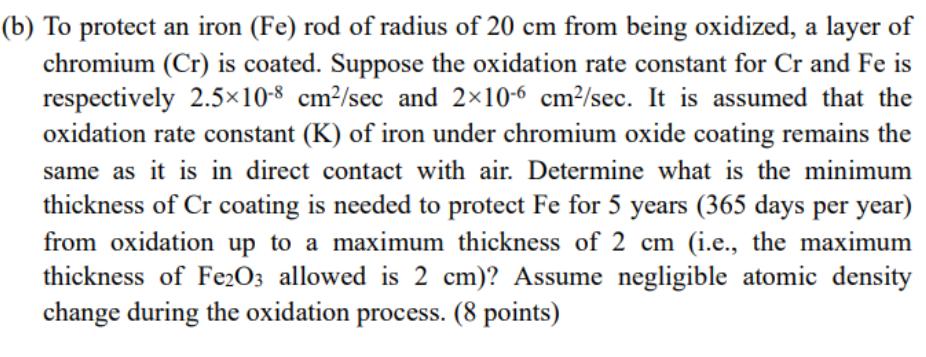
(b) To protect an iron (Fe) rod of radius of 20 cm from being oxidized, a layer of chromium (Cr) is coated. Suppose the oxidation rate constant for Cr and Fe is respectively 2.510-8 cm/sec and 2x10-6 cm/sec. It is assumed that the oxidation rate constant (K) of iron under chromium oxide coating remains the same as it is in direct contact with air. Determine what is the minimum thickness of Cr coating is needed to protect Fe for 5 years (365 days per year) from oxidation up to a maximum thickness of 2 cm (i.e., the maximum thickness of Fe2O3 allowed is 2 cm)? Assume negligible atomic density change during the oxidation process. (8 points)
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
To determine the minimum thickness of the chromium Cr coating needed to protect the iron Fe rod for 5 years while limiting the maximum thickness of Fe2O3 to 2 cm we can use Ficks second law of diffusi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


