Answered step by step
Verified Expert Solution
Question
1 Approved Answer
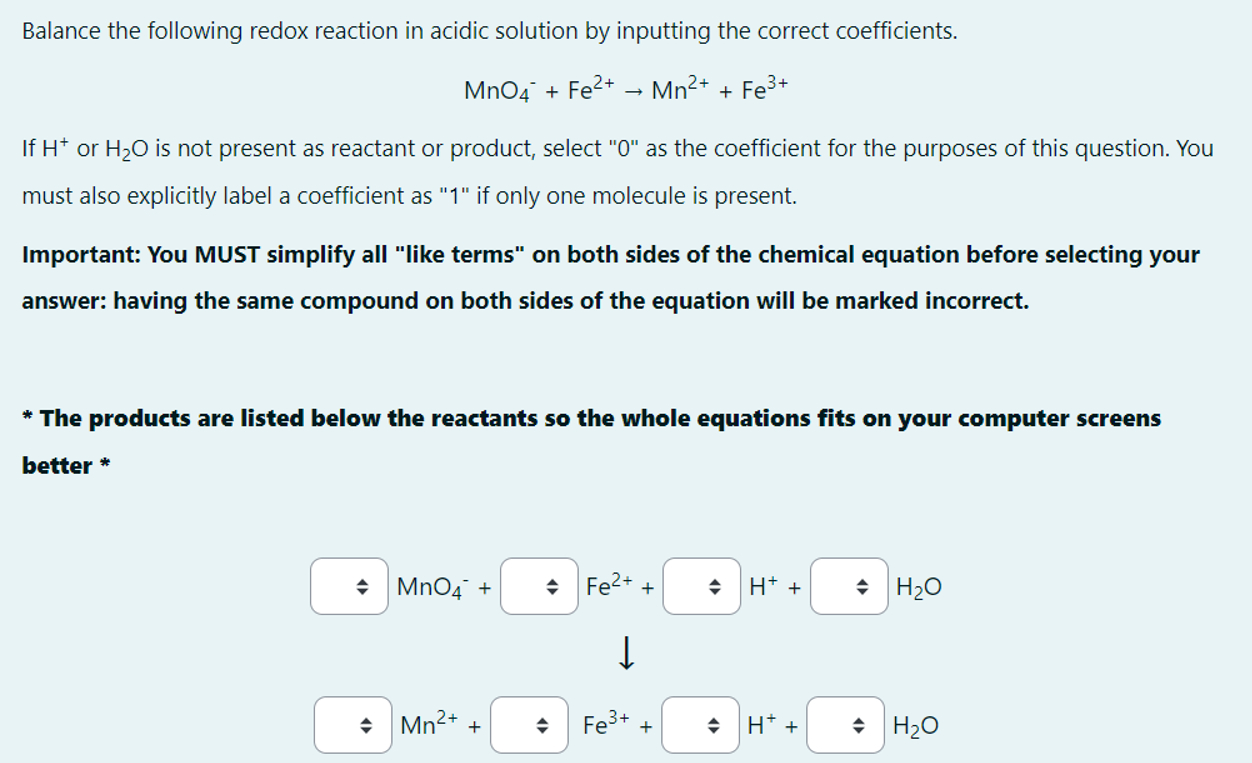
Balance the following redox reaction in acidic solution by inputting the correct coefficients. M n O 4 - + F e 2 + M n
Balance the following redox reaction in acidic solution by inputting the correct coefficients.
If or is not present as reactant or product, select as the coefficient for the purposes of this question. You
must also explicitly label a coefficient as if only one molecule is present.
Important: You MUST simplify all "like terms" on both sides of the chemical equation before selecting your
answer: having the same compound on both sides of the equation will be marked incorrect.
The products are listed below the reactants so the whole equations fits on your computer screens
better
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


