Answered step by step
Verified Expert Solution
Question
1 Approved Answer
based on the diagram (soybean oil extraction), please help me to solve the energy balance for every unit operation. (Basis 30 tan of soya bean
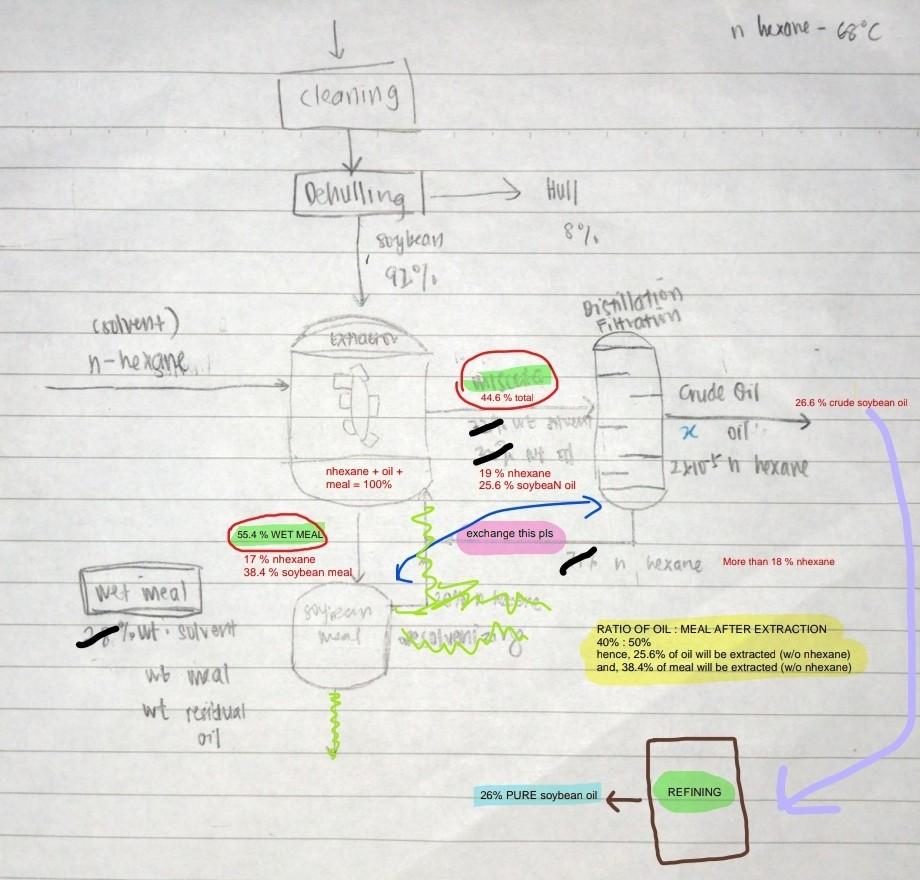
based on the diagram (soybean oil extraction), please help me to solve the energy balance for every unit operation. (Basis 30 tan of soya bean oil)
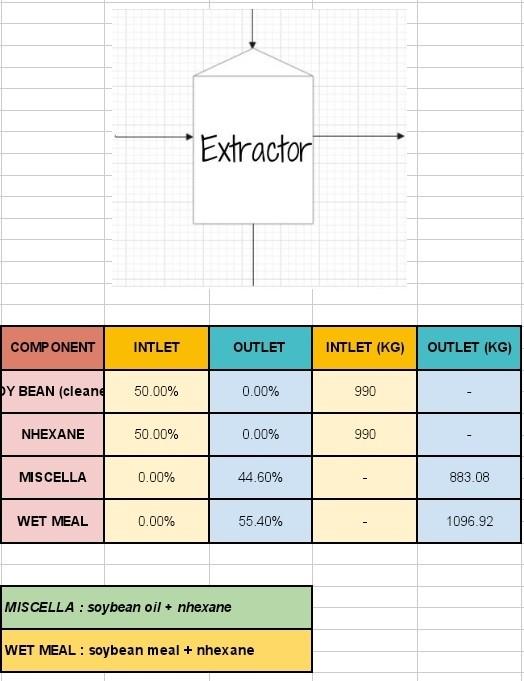

based on the information above, help me to solve general energy balance non reactive.
in hexane - 66C cleaning Dehulling > Hull 87 soybean 9197 Distillation Filtration csolvent) n-hexane traer th 44.6 % total 26.6 % crude soybean oil Grude Oil X Oil 2004 h hexane nhexane + oil + meal = 100% 19% nhexane 25.6 % soybean oil 55.4 % WET MEAL exchange this pls en hexane More than 18 % nhexane 17% nhexane 38.4 % soybean meal wet meal soupean 1, wt. suvert ma Ristorage sekulyar RATIO OF OIL : MEAL AFTER EXTRACTION 40%: 50% hence, 25.6% of oil will be extracted (w/o nhexane) and, 38.4% of meal will be extracted (w/o nhexane) wb weat wt residual 011 LU 26% PURE soybean oil REFINING Extractor COMPONENT INTLET OUTLET INTLET (KG) OUTLET (KG) DY BEAN (cleane 50.00% 0.00% 990 NHEXANE 50.00% 0.00% 990 MISCELLA 0.00% 44.60% 883.08 WET MEAL 0.00% 55.40% 1096.92 MISCELLA : soybean oil + nhexane WET MEAL : soybean meal + nhexane Energy Balance Assumptions: 1. All the units are operated at atmospheric pressure 2. There are no channge in Potential energy, Kinetic energy, and no shaft work 3. No heat loss surrounding 4. No enthalpy changes, no chemical reaction in the unit, and temperature constantStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


