Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chapter 10: Nucleophilic Substitution Reaction II The Kinetics of Solvolysis- SN1 Reaction Write Prelab: Purpose, Introduction, Procedures, and Table of Physical Constants Explanation: In

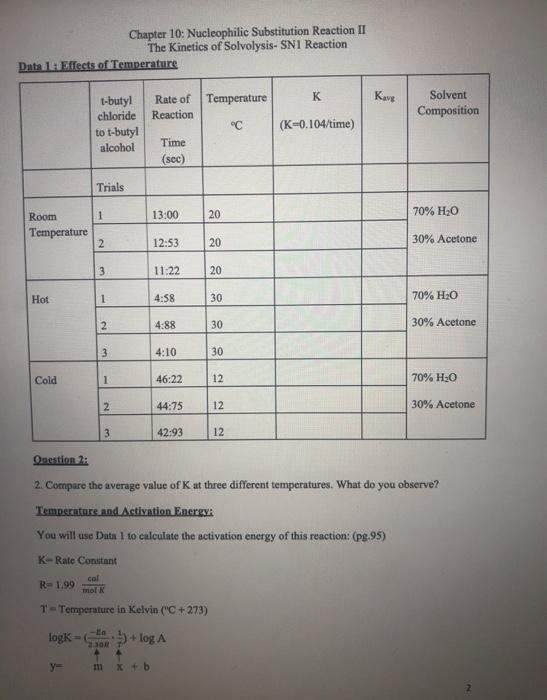
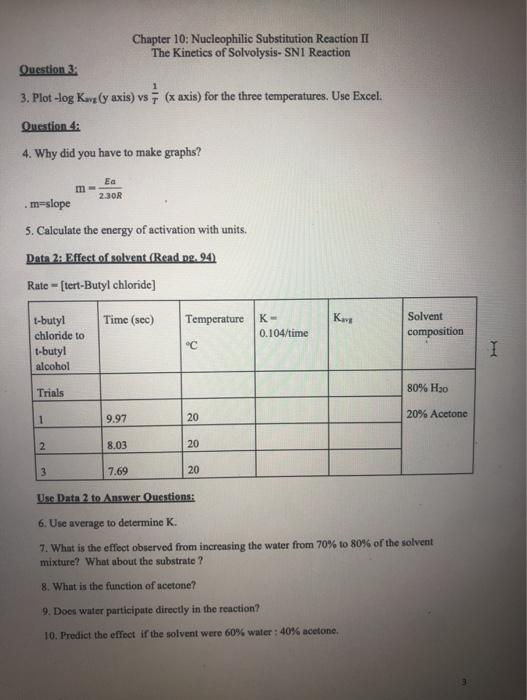
Chapter 10: Nucleophilic Substitution Reaction II The Kinetics of Solvolysis- SN1 Reaction Write Prelab: Purpose, Introduction, Procedures, and Table of Physical Constants Explanation: In the experiment, we are going to examine the rate of tert-Butyl alcohol+ Cr tert-Butyl chloride + HO HO in this reaction is a solvent and nucleophile. Sometimes, in an SN1 reaction the solvent acts as the nucleophile. This is called solvolysis. Factors which affect the rate of reaction: A. The concentration of the reactants B. The solvent C. The temperature Concentration of the Reactants: A. The reaction involves two separate steps. First the leaving group departs, and then the nucleophile attacks the carbocation. The first step is the rate-determining step. The rate law for the reaction is as follows: Rate = k [tert-butyl chloride] The rate law doesn't involve water so changing the concentration of the nucleophile has no effect on the rate The rate law does involve [tert-butyl chloride], so increasing the concentration of the substrate increases the rate. Likewise, decreasing the concentration of the substrate decreases the rate. Solvent: B. See explanation for A. Temperature: C. A rise in temperature should accelerate a bond breaking reaction. The rate determining step involves bond breaking. So, we would predict that an increase in temperature should result in production of more tert-butyl and thus increases the rate of the reaction. Question 1: 1. Write the mechanism of the reaction. Data 1: Effects of Temperature Room Temperature Hot Cold 1-butyl chloride to t-butyl alcohol Trials y= 1 2 3. 1 2 3 1 Chapter 10: Nucleophilic Substitution Reaction II The Kinetics of Solvolysis- SN1 Reaction 2 3 Rate of Temperature Reaction Time (sec) m x + b 13:00 12:53 11:22 4:58 4:88 4:10 46:22 44:75 42:93 20 20 T-Temperature in Kelvin ("C +273) logk=+log A 20 30 30 30 12 12 12 K (K-0.104/time) Kavg Solvent Composition 70% HO 30% Acetone 70% HO 30% Acetone Question 2: 2. Compare the average value of K at three different temperatures. What do you observe? Temperature and Activation Energy: You will use Data 1 to calculate the activation energy of this reaction: (pg.95) K-Rate Constant col R=1.99 mol K 70% HO 30% Acetone Question 3: 3. Plot-log Kang (y axis) vs (x axis) for the three temperatures. Use Excel. Question 4: 4. Why did you have to make graphs? t-butyl chloride to .m-slope 5. Calculate the energy of activation with units. Data 2: Effect of solvent (Read pg. 94) Rate-[tert-Butyl chloride] t-butyl alcohol Trials 1 2 Ea 2.30R 3 Chapter 10: Nucleophilic Substitution Reaction II The Kinetics of Solvolysis- SN1 Reaction Time (sec) 9.97 8.03 7.69 Temperature C 20 20 20 K- 0.104/time Kava Solvent composition 9. Does water participate directly in the reaction? 10. Predict the effect if the solvent were 60% water: 40% acetone. 80% H0 20% Acetone Use Data 2 to Answer Questions: 6. Use average to determine K. 7. What is the effect observed from increasing the water from 70% to 80% of the solvent mixture? What about the substrate? 8. What is the function of acetone? I
Step by Step Solution
★★★★★
3.50 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Question we observed that as temperature is higher than rate of react...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


