Question
You are given three bottles labeled A, B, and C. Each bottle contains a white solid. The solid in bottle A melts at 801
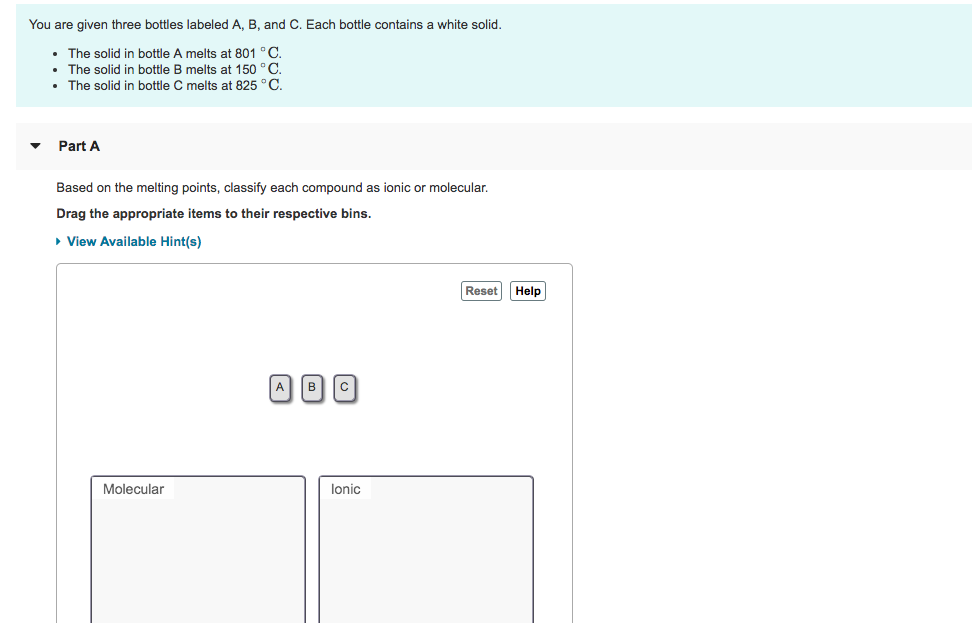
You are given three bottles labeled A, B, and C. Each bottle contains a white solid. The solid in bottle A melts at 801 C. The solid in bottle B melts at 150 C. The solid in bottle C melts at 825 C. Part A Based on the melting points, classify each compound as ionic or molecular. Drag the appropriate items to their respective bins. View Available Hint(s) Molecular A B C lonic Reset Help
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Based on the melting points classify each compound as ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials Of Business Statistics
Authors: Bruce Bowerman, Richard Connell, Emily Murphree, Burdeane Or
5th Edition
978-1259688867, 1259688860, 78020530, 978-0078020537
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



