Answered step by step
Verified Expert Solution
Question
1 Approved Answer
basic 1 for chemical engineering ihe following two reactions are carried in a pressurized gas phase reactor at 2.8 bar: 2A+2BC+D2AF+G The equilibrium constants for
basic 1 for chemical engineering 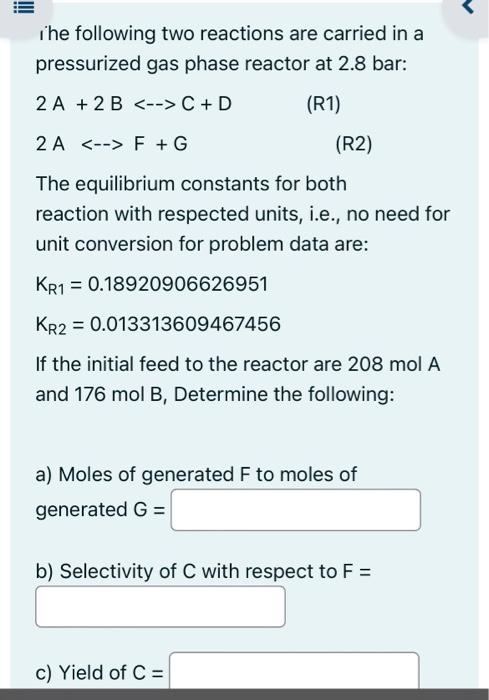


ihe following two reactions are carried in a pressurized gas phase reactor at 2.8 bar: 2A+2BC+D2AF+G The equilibrium constants for both reaction with respected units, i.e., no need for unit conversion for problem data are: KR1=0.18920906626951KR2=0.013313609467456 If the initial feed to the reactor are 208molA and 176molB, Determine the following: a) Moles of generated F to moles of generated G= b) Selectivity of C with respect to F= c) Yield of C= Yield of C= d) For this system, increasing the pressure would: 1. Decrease yield of C 2. No effect on the yield of C 3. Increase yield of C 4. Need more information Correct answer is Discuss this in the submitted solution for more grades d) For this system, using inert in the initial feed would: 1. Decrease yield of C 2. No effect on the yield of C 3. Increase yield of C 4. Need more information Correct answer is vould: 1. Decrease yield of C 2. No effect on the yield of C 3. Increase yield of C 4. Need more information Correct answer is Discuss this in the submitted solution for more grades d) For this system, using inert in the initial feed would: 1. Decrease yield of C 2. No effect on the yield of C 3. Increase yield of C 4. Need more information Correct answer is Discuss this in the submitted solution for more grades 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


