Answered step by step
Verified Expert Solution
Question
1 Approved Answer
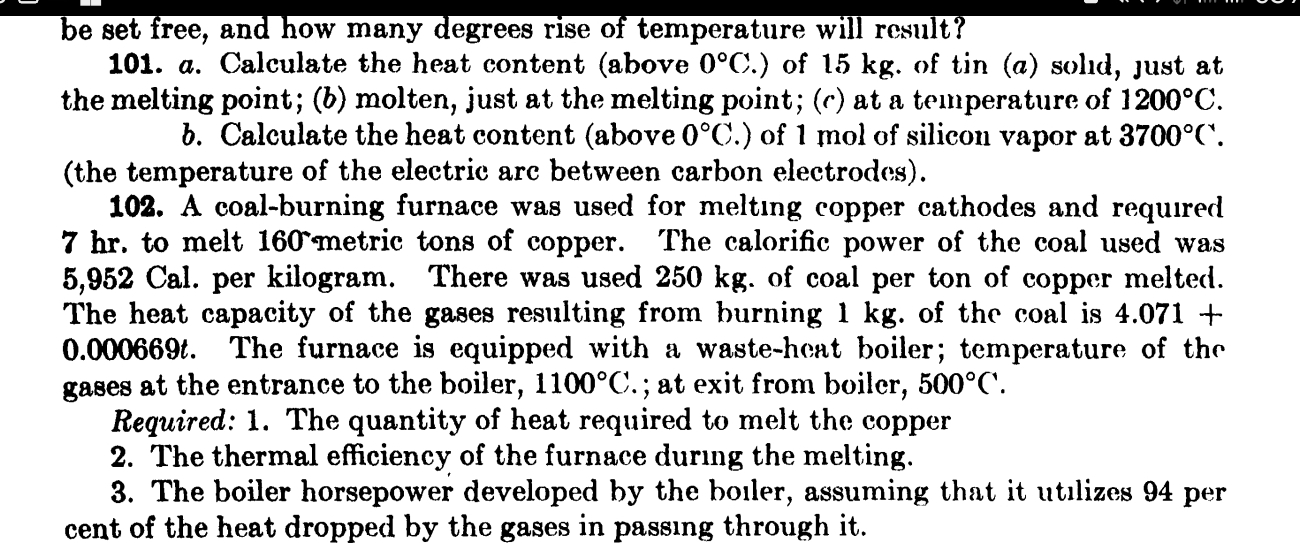
be set free, and how many degrees rise of temperature will result? 1 0 1 . a . Calculate the heat content ( above 0
be set free, and how many degrees rise of temperature will result?
a Calculate the heat content above of of tin a sold, just at the melting point; molten, just at the melting point; at a temperature of
b Calculate the heat content above of mol of silicon vapor at the temperature of the electric arc between carbon electrodes
A coalburning furnace was used for melting copper cathodes and required to melt metric tons of copper. The calorific power of the coal used was Cal. per kilogram. There was used of coal per ton of copper melted. The heat capacity of the gases resulting from burning of the coal is The furnace is equipped with a wasteheat boiler; temperature of the gases at the entrance to the boiler, ; at exit from boiler,
Required: The quantity of heat required to melt the copper
The thermal efficiency of the furnace during the melting.
The boiler horsepower developed by the boller, assuming that it utilizes per cent of the heat dropped by the gases in passing through it
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


