Question
The voltages versus Li measured in Li-Co-O system are shown in the ternary phase diagram. The Gibbs free energies of pure substances (unit kJ/mol
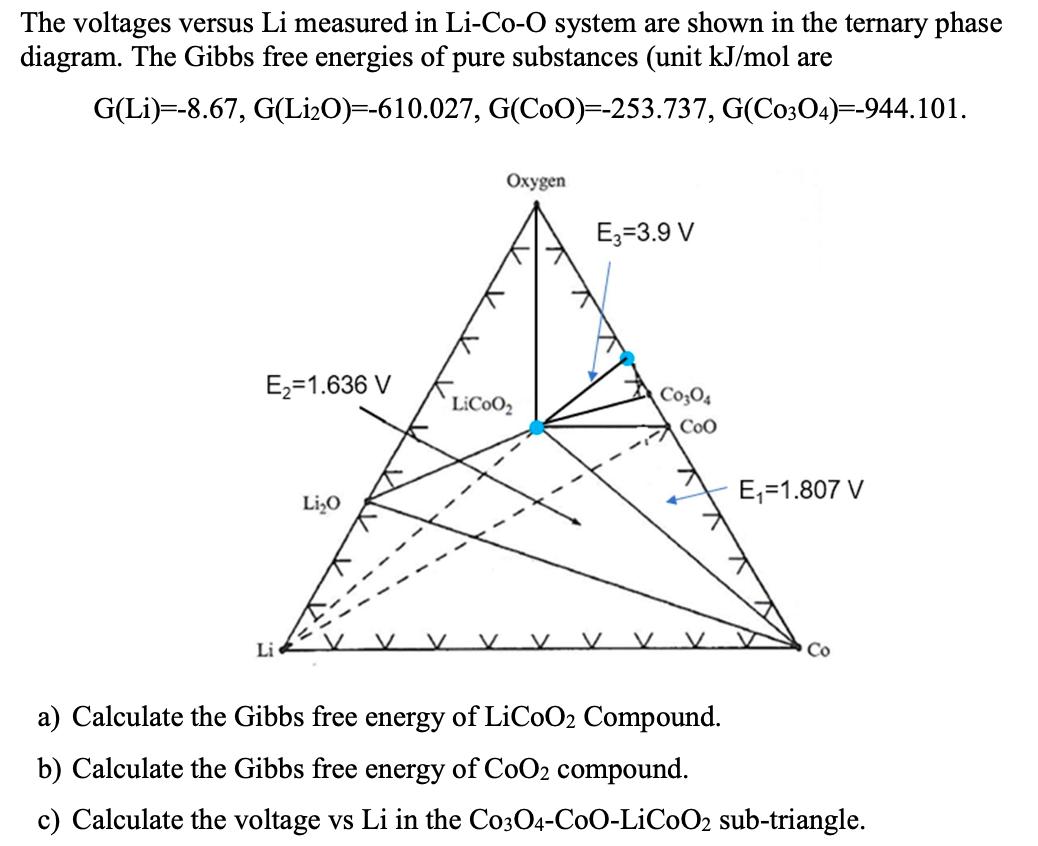
The voltages versus Li measured in Li-Co-O system are shown in the ternary phase diagram. The Gibbs free energies of pure substances (unit kJ/mol are G(Li) -8.67, G(LiO)=-610.027, G(COO)=-253.737, G(C0304)=-944.101. E=1.636 V Li LiO Oxygen LiCoo E3-3.9 V V C0304 Coo E=1.807 V Co a) Calculate the Gibbs free energy of LiCoO2 Compound. b) Calculate the Gibbs free energy of CoO2 compound. c) Calculate the voltage vs Li in the C0304-C0O-LiC0O2 sub-triangle.
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 The ternary phase diagram of the LiCoO system shows the voltages versus Li measured ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



