Question
Below are the concentration used in the lab. Please help me do mol fraction for the following table using the eqn given. 0.100 M Cu2+
Below are the concentration used in the lab. Please help me do mol fraction for the following table using the eqn given.
0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+
0.100 M EDTA 3.98 103 M SCN 0.400 M en
The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA.
The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+)
The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
Could you please do a sample calulation for me
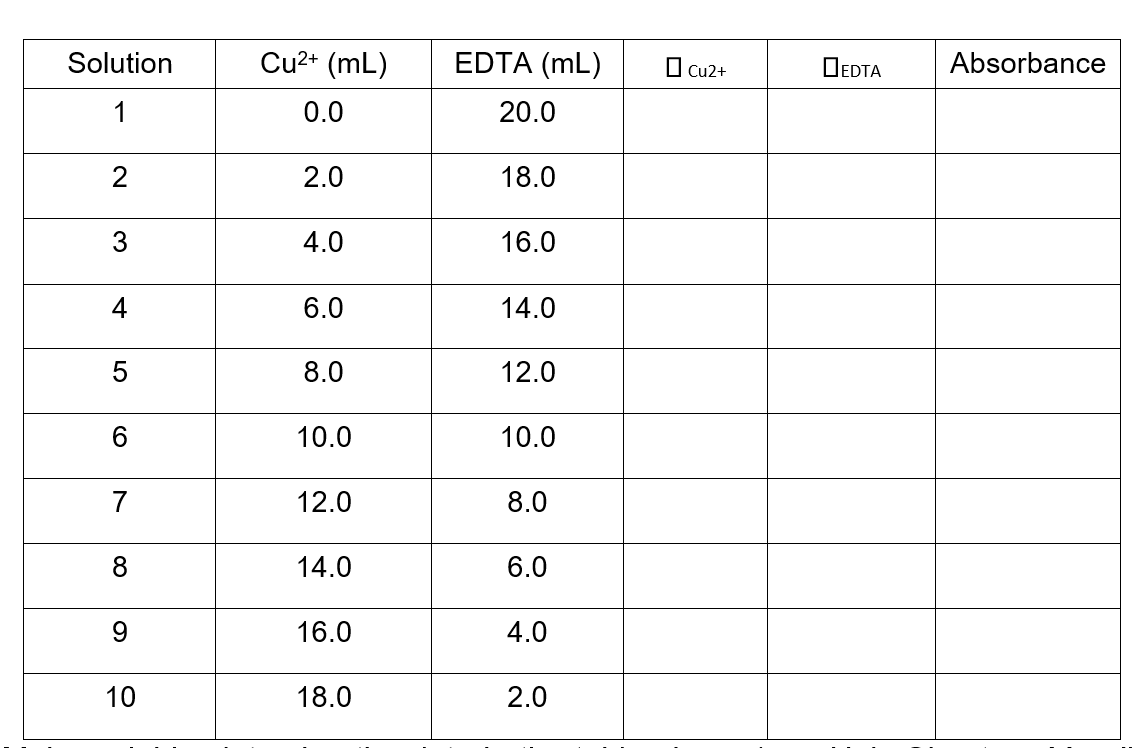 0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+ 0.100 M EDTA 3.98 103 M SCN 0.400 M en The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA. The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+) The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
0.100 M Cu2+ 3.98 103 M Fe3+ 0.400 M Ni2+ 0.100 M EDTA 3.98 103 M SCN 0.400 M en The mole fraction () is a ratio of the various species in solution. One of the solutions in this experiment is a two-component mixture of Cu2+ and EDTA. The mole fraction for EDTA would therefore be expressed as: EDTA = (# mol EDTA) / (# mol EDTA + # mol Cu2+) The mole fraction for copper(II) would then be: Cu2+ = 1 EDTA
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


