Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Below is a Table showing the resuits from a Jar test conducted to determine the optimal dosage of alum to treat a specific surface
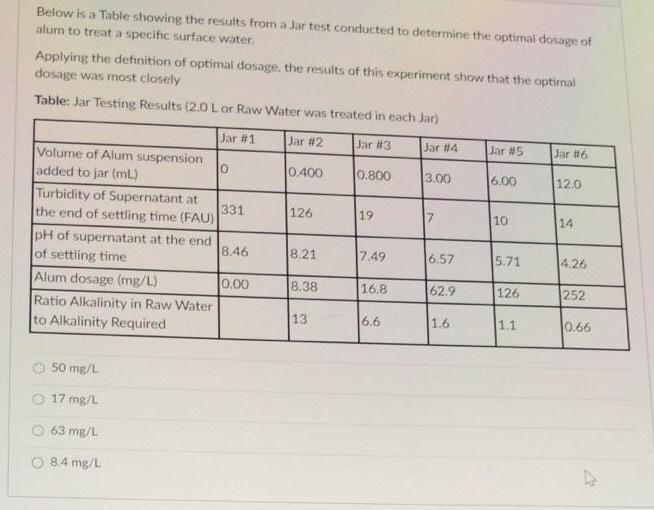
Below is a Table showing the resuits from a Jar test conducted to determine the optimal dosage of alum to treat a specific surface water. Applying the definition of optimal dosage, the results of this experiment show that the optimal dosage was most closely Table: Jar Testing Results (2.0 Lor Raw Water was treated in each Jar) Jar #1 Jar #2 Jar #3 Jar #4 Jar #5 Jar #6 Volume of Alum suspension added to jar (mL) 0.400 0.800 3.00 6.00 12.0 Turbidity of Supernatant at 331 the end of settling time (FAU) 126 19 10 14 pH of supernatant at the end of settling time Alum dosage (mg/L) Ratio Alkalinity in Raw Water to Alkalinity Required 8.46 8.21 7.49 6.57 5.71 4. 26 0.00 8.38 16.8 62.9 126 252 13 6.6 1.6 1.1 0.66 O 50 mg/L O 17 mg/L 63 mg/L 8.4 mg/L
Step by Step Solution
★★★★★
3.47 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
63 mgL option C reason Because the optimal range of pH of alum is between 6 to 73 For 629 mgL ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


