Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Below we present the MO diagram of ML4 molecule with a C4 point group, as well as detailed information of each MO. Here L
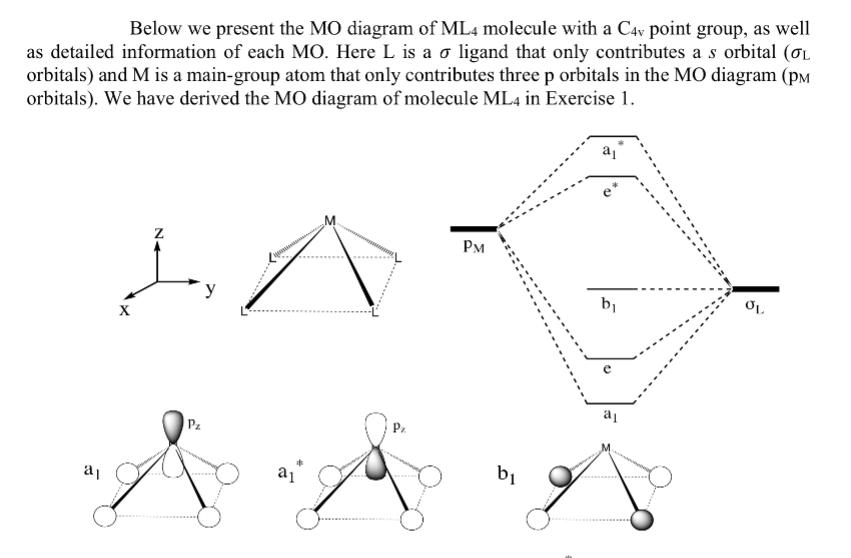
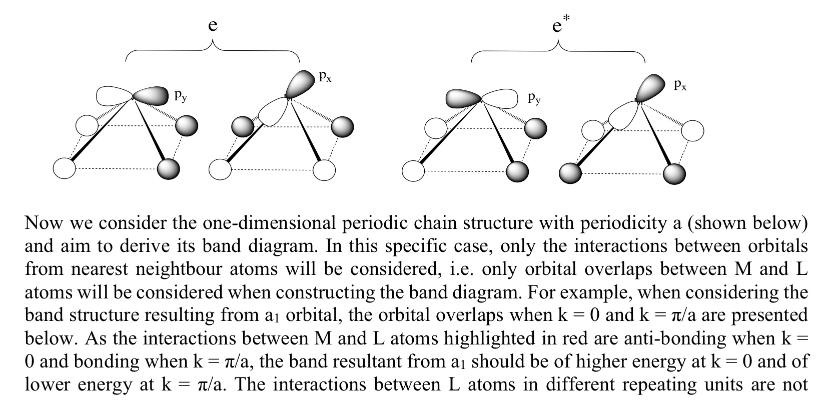
Below we present the MO diagram of ML4 molecule with a C4 point group, as well as detailed information of each MO. Here L is a o ligand that only contributes a s orbital (OL orbitals) and M is a main-group atom that only contributes three p orbitals in the MO diagram (PM orbitals). We have derived the MO diagram of molecule ML4 in Exercise 1. a Pz Pz. PM b b OL e Px Py R D Px Now we consider the one-dimensional periodic chain structure with periodicity a (shown below) and aim to derive its band diagram. In this specific case, only the interactions between orbitals from nearest neightbour atoms will be considered, i.e. only orbital overlaps between M and L atoms will be considered when constructing the band diagram. For example, when considering the band structure resulting from a orbital, the orbital overlaps when k = 0 and k = n/a are presented below. As the interactions between M and L atoms highlighted in red are anti-bonding when k = 0 and bonding when k = /a, the band resultant from a should be of higher energy at k = 0 and of lower energy at k T/a. The interactions between L atoms in different repeating units are not =
Step by Step Solution
★★★★★
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 b I base i Molecular Orbital Diagram for FeI 6 4 hexaiodidoferrateII complex The valence orbitals of Fe 2 will be 3d 4s 4p and 3d orb...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


