Answered step by step
Verified Expert Solution
Question
1 Approved Answer
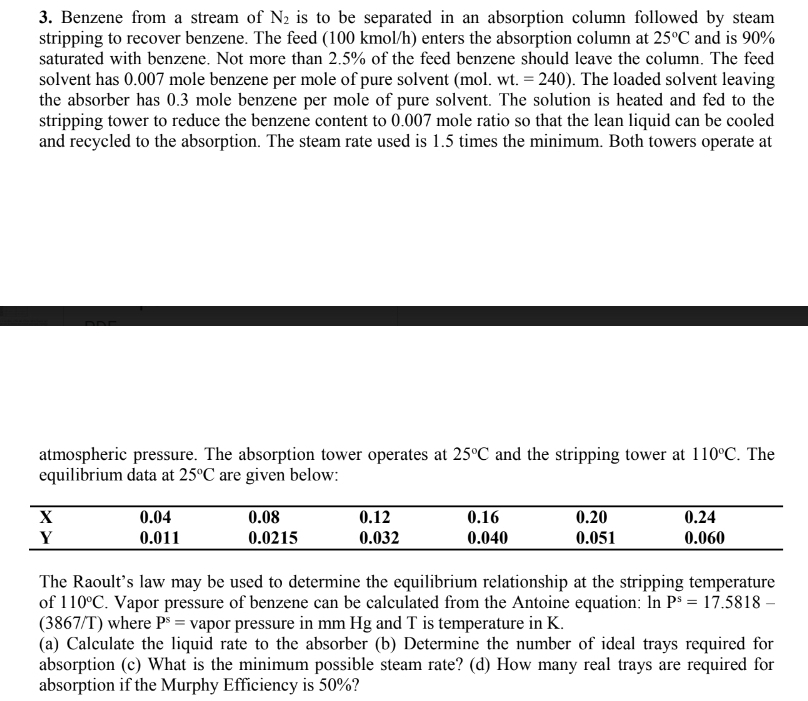
Benzene from a stream of N 2 is to be separated in an absorption column followed by steam stripping to recover benzene. The feed (
Benzene from a stream of is to be separated in an absorption column followed by steam
stripping to recover benzene. The feed kmo enters the absorption column at and is
saturated with benzene. Not more than of the feed benzene should leave the column. The feed
solvent has mole benzene per mole of pure solvent The loaded solvent leaving
the absorber has mole benzene per mole of pure solvent. The solution is heated and fed to the
stripping tower to reduce the benzene content to mole ratio so that the lean liquid can be cooled
and recycled to the absorption The steam rate used is times the minimum. Both towers operate at
atmospheric pressure. The absorption tower operates at and the stripping tower at The
equilibrium data at are given below:
The Raoult's law may be used to determine the equilibrium relationship at the stripping temperature
of Vapor pressure of benzene can be calculated from the Antoine equation:
T where vapor pressure in and is temperature in
a Calculate the liquid rate to the absorber b Determine the number of ideal trays required for
absorption c What is the minimum possible steam rate? d How many real trays are required for
absorption if the Murphy Efficiency is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


