Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Benzoic acid and its salts have been found useful as ingredients of a number of industrial products, among which are adhesive, rubber latex, and soap.
Benzoic acid and its salts have been found useful as ingredients of a number of industrial products, among which
are adhesive, rubber latex, and soap.
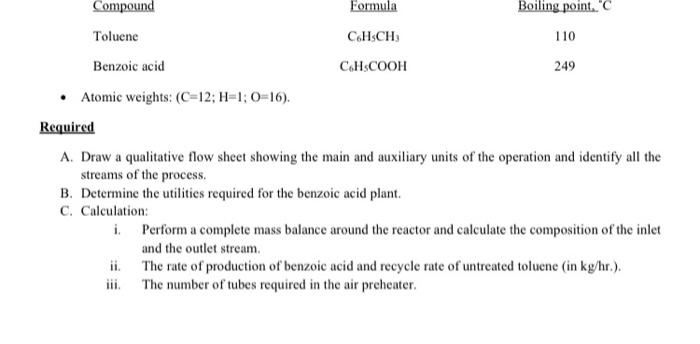
Benzoic acid is being produced by the vapor phase oxidation of toluene in a continuous flow reactor, fitted with a fixed bed of the catalyst. The toluene is fed to a vertical-tube vaporizer, which uses superheated steam. Air for oxidation is passed inside the tubes of a horizontal-tube preheater, where it is heated from 20 C to 90 C by hot oil flowing outside the tubes counter-currently to the air flow. Both hot air and the toluene vapors are mixed before introducing into the fixed-bed reactor. The reaction is only 85% complete and therefore the vapors coming out from the reactor are passed to a cooler condenser where non-condensable gases are disposed. The liquids exit from the condenser are phase-separated in a gravity settler.
The following information is available:
The feed rate of toluene to the vaporizer is 200 kg/hr.
Excess air used for oxidation is 50%.
Percent recovery of product and unreacted toluene is 100%.
Overall heat transfer coefficient in the air preheater is 6 W/m2.K.
Tubes used in the preheater are 50 mm in diameter and 2 m long.
Hot oil is fed to the air preheater at 250 C and leaves at 195 C.
Air composition: N2 (79 mole %) and O2 (21 mole %); its molecular weight is 29; its specific heat is 1.1
kJ/kgK.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


