Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Benzoic acid is an important organic chemical that is used for the production of phenol, sodium benzoate, benzoyl chloride, benzonitrile and other esters. The usual
Benzoic acid is an important organic chemical that is used for the production of phenol, sodium benzoate, benzoyl chloride, benzonitrile and other esters. The usual method of production is by the liquid phase oxidation of toluene at a temperature between and and pressure in the range to bar. The stoichiometric reaction is
The project will consist of the design of a plant to produce tonnesyear of benzoic acid minimum purity assuming an operating period of hours on stream. The process will similar to the present industrial practice except that the side reactions, which produce some benzaldehyde and carbon dioxide will be ignored.
The following parts will be undertaken.
a A complete material and energy balance on a basis of kmol toluene entering reactor
b An outline design of the reactor
c Design and specifications for various heat exchangers, pumps, and other plant items
d An economic assessment of the plant.
e Process improvement ideas and evaluation.
Feed consisting of fresh and recycled liquid toluene is supplied to the reactor together with air that has been compressed to a pressure of bar. The molar flowrate of air employed is kmol per kmol toluene and the reaction takes place at and the temperature is controlled at by cooling using an external jacket and internal coil to remove the exothermic heat of reaction.
The contents of the reactor are perfectly mixed and the gross toluene feed rate is adjusted until, under steady conditions, of the toluene is converted to benzoic acid. Benzoic acid has a low vapour pressure at the reaction temperature and stays entirely in the liquid phase. Some toluene and water leave the reactor as vapour along with the nitrogen and unreacted oxygen. The mol fraction of oxygen in that stream is and the partial pressures exerted by the toluene and water are proportional to their mol fractions in the liquid that leaves the reactor.
The gasvapour mixture from the reactor passes to a cooler condenser where most of the toluene and water are condensed. The cooler condenser operates at the same temperature as the reactor and nitrogen and oxygen and residual vapour are vented to the atmosphere at a temperature of In the vented gas, the partial pressures of toluene and water are equal to their saturated vapour pressures at which is also the temperature of the outlet condensate. The condensate flows to a separator where the toluene and water separate completely into two liquid phases. The water is discarded and the toluene is returned to the reactor.
The crude liquid product from the reactor passes to a distillation column operated at atmosphere pressure. The reduction in pressure causes onethird of the feed to be vaporised and the feed enters the column at about The distillate contains toluene and water in the same molar proportions as those in the feed together with a small amount of benzoic acid and is at a temperature of of the toluene is recovered in the distillate, which flows to a separator to remove the water from the toluene rich stream. The benzoic acid in the distillate stays dissolved in the toluene as phase separation takes place. The toluenerich mixture is recycled back to the reactor and the water layer is again discarded.
The bottom product from the distillation column contains not less than mol benzoic acid and is at a temperature of It is cooled to before being passed to a solidification drum. This device is watercooled and produces a continuous sheet of solid benzoic acid. The solid material is broken up into flakes and these pa
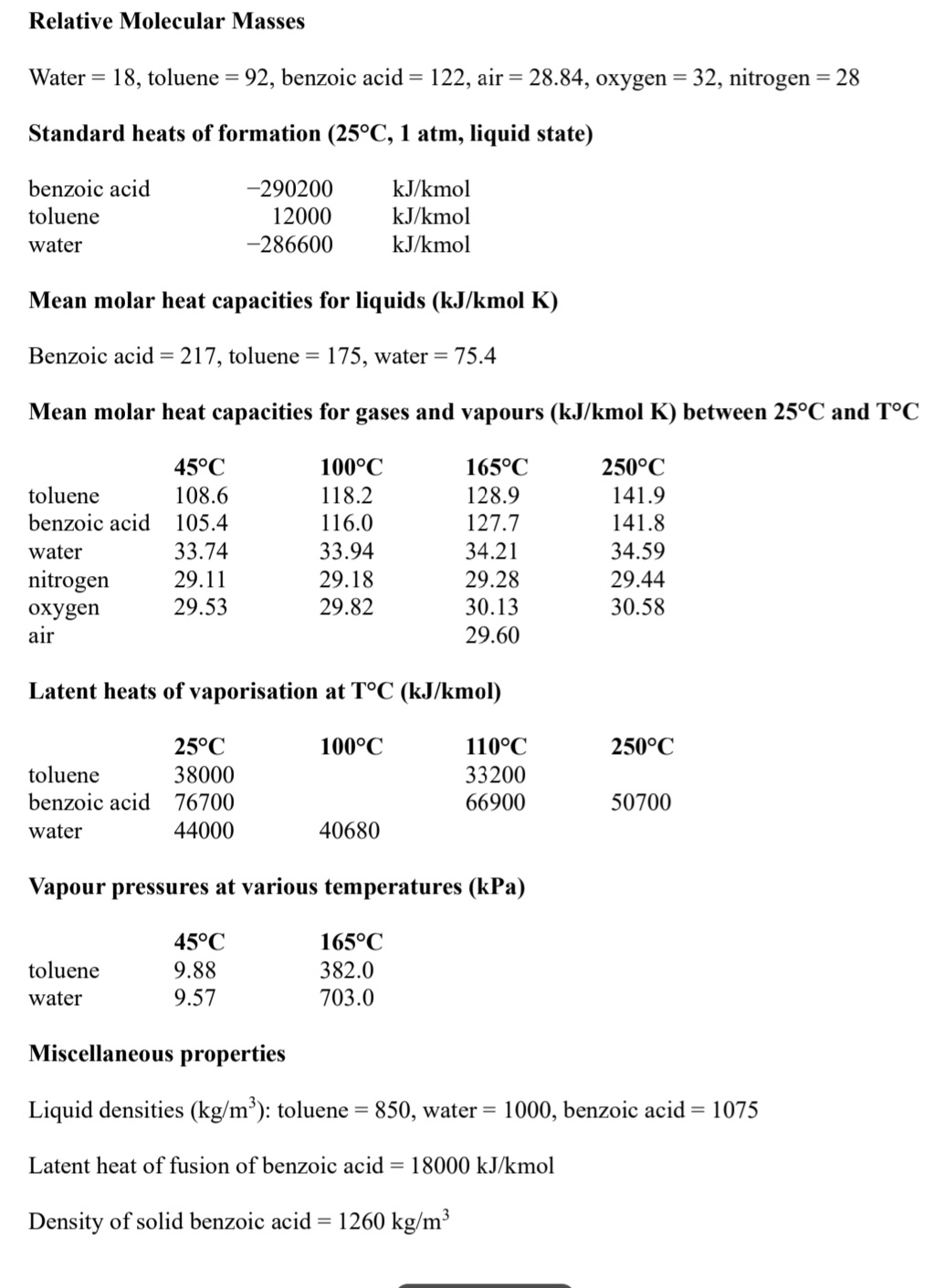
Relative Molecular Masses
Water toluene benzoic acid air oxygen nitrogen
Standard heats of formation liquid state ss to the packaging section of the plant.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


