Question
()-Berkeleyamide D is a natural product from Penicillium rubrum bacteria which is a micromolar inhibitor of the potential drug target MMP-3. This following synthetic sequence
(±)-Berkeleyamide D is a natural product from Penicillium rubrum bacteria which is a micromolar inhibitor of the potential drug target MMP-3.
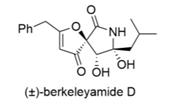
This following synthetic sequence is adapted from a recently reported biomimetic synthesis.
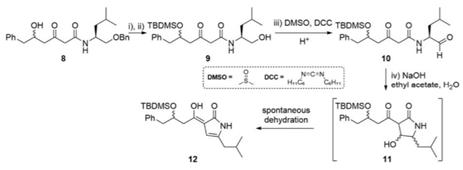
a) Propose reagents and their order for steps i) and ii) to convert 8 into 9. (2 Marks)
b) Redraw step iv) in the retrosynthetic direction to show the disconnection of compound 11 back to the synthons that correspond to compound 10. (3 Marks)
c) Propose two different reasonable first disconnections (i.e. only one step backward) for the retrosynthesis of compound 8 from simpler starting materials. Show all your working, including the bond broken, synthons, and corresponding reagents. Also, state one potential issue of selectivity or stability arising from one of your disconnection choices. (8 Marks)
d) The oxidation of the primary alcohol in 9 to an aldehyde in 10 is achieved using the Pfitzner-Moffatt oxidation, using DMSO and DCC as reagents (closely related to the Swern oxidation). Provide a plausible mechanism for this transformation, starting with DMSO and DCC reacting with each other. (4 Marks)
Ph NH OH H (2)-berkeleyamide D
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
All mechanisms ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


